Geoscience Reference
In-Depth Information
Front view
Side view
T. tepidum:L
T. tepidum:M
R. sphaeroids:L
R. sphaeroids:M
R. viridis:L
R. viridis:M
T. elongatus:D1
T. elongatus:D2
S. elongatus:psaA
S. elongatus:psaB
Type II
Type I
Type I
PS II
PS I
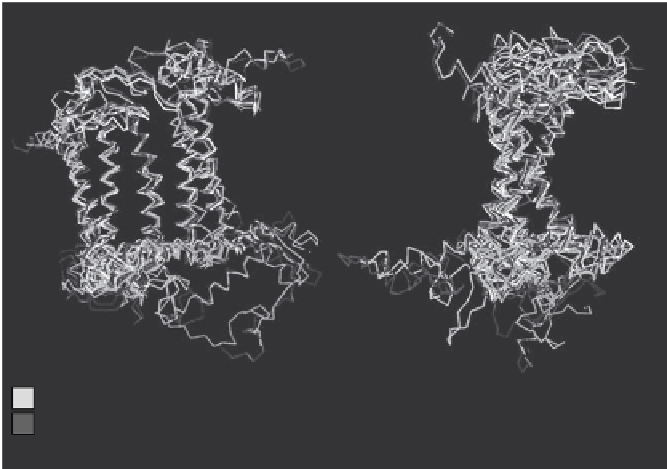
Figure 3.4. Comparison of the structures of the photosynthetic reaction center proteins.
Also indicated is whether the proteins are type I or type II anoxygenic photosynthetic
reaction centers, or whether they are PsII or PsI from oxygenic phototrophic cyanobacteria.
Borrowed with slight modification from sadekar et al. (2006). reproduced with permission.
see
plate 6
for a color version.
and differences in structure between the different reaction center pro-
teins can be compared with certainty. The logic in making such com-
parisons is that proteins with similar structures are more closely related
evolutionarily. So, when structures of the proteins from PSI, PSII, and
a color version), there is a remarkable similarity, particularly in the mid-
dle regions of the protein where it is embedded in a membrane within
the cell. This is good evidence that all of the reaction center proteins are
related to one another. With further analysis of these protein structures,
Sadekar, Raymond, and Blankenship concluded, consistent with Bob
Blankenship's original suggestion, that anoxygenic photosynthetic
Type 1 and Type 2 proteins are ancestral to PSI and PSII.
You can probably guess where this is going. A logical explanation for
all of these observations is that the reaction centers PSI and PSII were
derived from the Type 1 and Type 2 reaction centers already existing
among anoxygenic photosynthetic bacteria. Somehow, and the details