Geoscience Reference
In-Depth Information
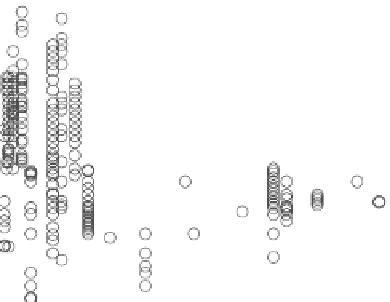
GOE
12
8
4
0
-4
-8
1500
1700
1900
2100
2300
2500
2700
2900
Age (millions of years)
Figure 8.3. Isotopic composition of inorganic carbon showing the lomagundi Isotope event
spanning from about 1950 to 2300 million years ago. Also shown is the gOe.
which it is formed. This means that the inorganic carbon remaining has
less C-12, or in other words, it becomes enriched in C-13. The more or-
ganic carbon we remove from the oceans, the more the inorganic carbon
remaining in the oceans will become enriched in C-13. We can see right
away that if we have extra-high rates of organic matter removal from the
ocean, we should generate extra-large C-13 enrichments in marine inor-
ganic carbon.
We can measure the ratio of C-13 to C-12 atoms in organic matter
from old rocks, as Minik Rosing did in rocks from Isua, Greenland
(
chapter 6)
, and since inorganic carbon is also removed as limestones
(and shells after the evolution of animals), we can measure the ratio of
C-13 to C-12 in inorganic carbon too. Thus, we can assemble a record
of the C-13 to C-12 ratio of both organic carbon and inorganic carbon
through time. If you remember from
chapter 6,
we generally discuss
these carbon isotope ratios as δ
13
C values, as we will here.
Now let's look at the data (
ig. 8.3)
and focus on the time of the GOE
(remember, this was around 2.30 to 2.35 billion years ago). Indeed,
δ
13
C of inorganic carbon becomes highly elevated around this time,
and this period of elevated values has been dubbed the Lomagundi
isotope excursion. By all appearances, it is the biggest carbon isotope