Geoscience Reference
In-Depth Information
Thus far, we have considered melting temperatures as if
they were unaffected by pressure. In fact, for mantle rock
there is a strong change of dry solidus temperature with
pressure. d
T
/d
P
is positive for the dry solidus of most key
silicate minerals of the Earth's mantle (e.g. Fig. 5.6) and
for the
garnet peridotite
composition (this is equivalent to
an
ultramafic rock
with
c
.90 percent of Fe- and Mg-bearing
minerals) that best seems to satisfy constraints for mean
mantle composition.
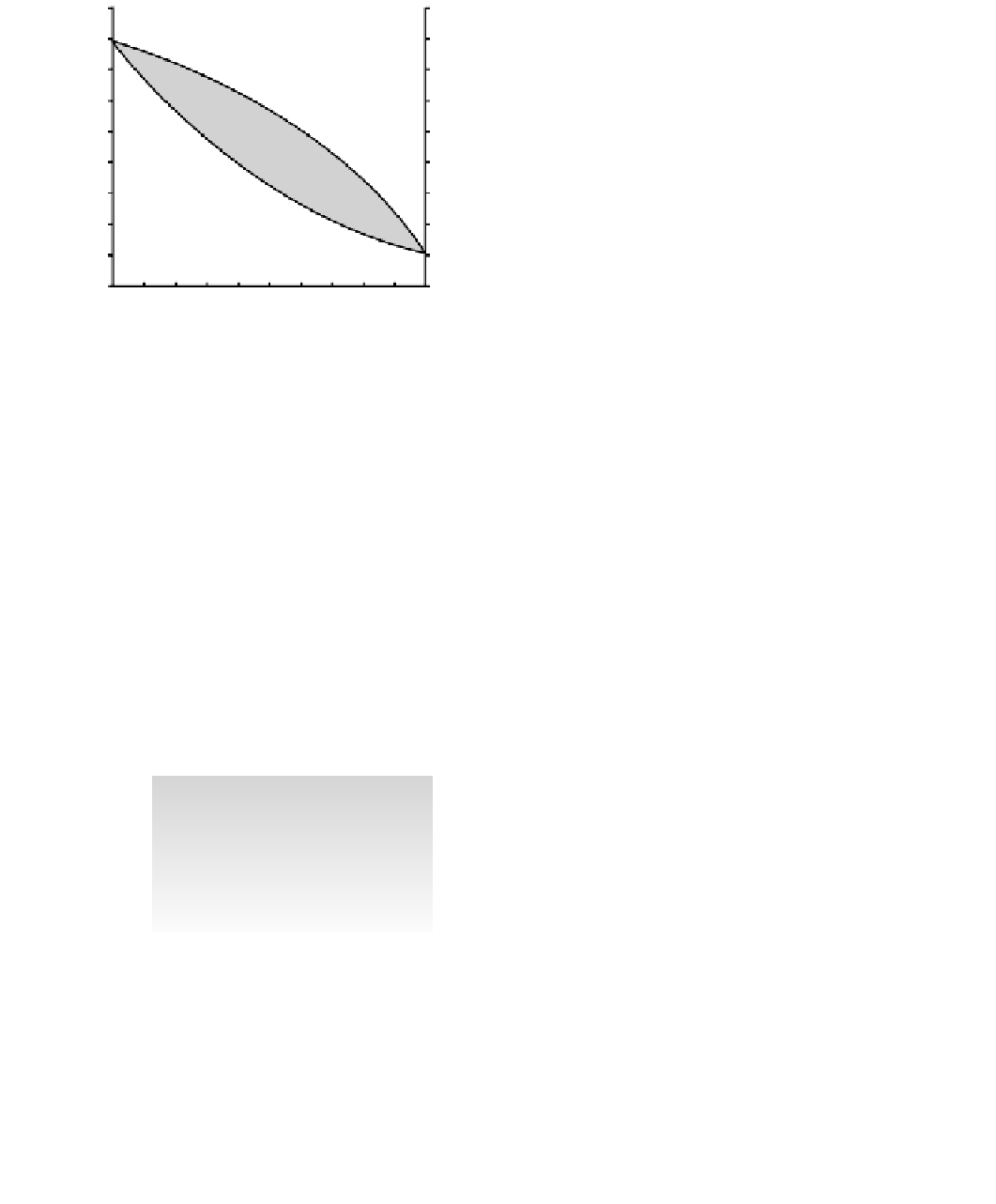
richer in Fe
2
than Mg
2
. As melting proceeds, the whole
melt progressively enriches in Mg
2
until it matches
the initial 50 : 50 mixture and melting of the initial solid
volume has become total at the liquidus. Experiments over
a range of initial compositions enable us to define a
phase
diagram
showing the range of solidus and liquidus appro-
priate to a whole solid solution series. Similar principles
govern the behavior of binary or ternary mixtures of
mineral phases.
5.1.3
Water, melting, and the terrestrial water cycle
2000
At 1 atm P
Water exerts a profound influence on both the melting
point (Fig. 5.9c) and strength of crustal and mantle rocks.
The presence of H
2
O in silicate melts is thought to cause
depolymerization by breaking the Si-O-Si bonds, leading
to the marked decreases in viscosity and melting tempera-
ture observed experimentally. For example, in order to
give a 20 percent melt fraction, the temperature of
anhydrous granite at 10 kbar pressure has to be about
900
Liquid silicate melt
1800
1600
Ol + liquid
Solid
olivine
1400
C; the addition of 4 percent by weight of water
decreases the required temperature to about 600
1200
C.
For basalt, the effect is even more startling for the positive
gradient of the dry solidus noted above is reversed and
at Moho depths of 35 km the saturated wet solidus
temperature is reduced from
c
.1150
50
Mg
2
SiO
4
Forsterite
Fe
2
SiO
4
Fayalite
Weight %
Fig. 5.8
Phase relations in the olivine solid solution series at
1 atm pressure.
C to 650
C.
Temperature (
°
C)
(a)
(b)
Temperature (
°
C)
1000
1200
1400
1000
1200
1400
0
0
Adiabatic upwelling
in convection limb or
stretched mantle
Solidus
Geotherm
50
50
Melt
Solidus
Mantle is heated,
geotherms increase
gradient, melting
occurs
100
100
Path
Path
2
1
Temperature (
°
C)
1200
1400
(c)
1000
0
(a) The situation in the rising limb of a major convection cell
under a midocean ridge or in stretched lithosphere.
(b) Mantle heating above a plume head causes geotherms to
intersect solidus.
(c) The asthenosphere above a subduction zone may melt if
there is sufficient flux of water from mineral dehydration
reactions, especially the breakdown of serpentinite minerals.
100
Solidus
Water acts as
a flux to lower
the melting
temperature
of mantle rock
200
1.
2.
Fig. 5.9
Various scenarios for the production of melt from mantle rocks.





Search WWH ::

Custom Search