Geoscience Reference
In-Depth Information
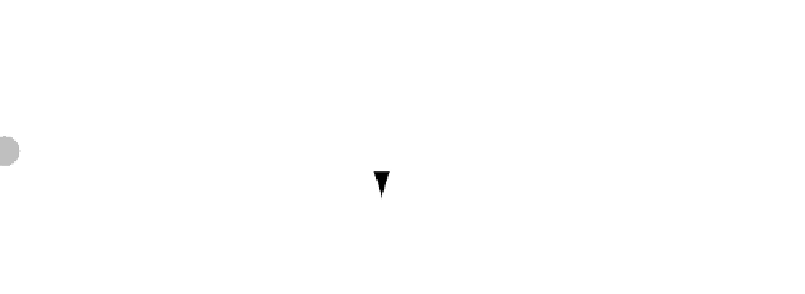
Fig. 4.28
Experimental double diffusive convection. At the beginning of the experiment the pale-colored saline water at the base of the image
underlay cold freshwater stably as a continuous layer. As it was heated from below, rapid heat diffusion lowered its density below that of the
freshwater. The less dense basal layer deformed into narrow ascending “fingers.” In reverse (view image upside down) this is how outflowing
warm, salty Mediterranean waters may react to cooling by the Atlantic. Image shows open top
Hele-Shaw cell
, length 800 mm, height 50 mm.
Mediterranean warm saline outflow into the Atlantic. The
situation leads to enhanced mixing by convection, some-
times called
double-diffusive convection
, at much greater
rates than mixing by molecular diffusion. In this process,
more rapid heat diffusion across zones of thermal contact
cause the stable stratification to break down. In our exam-
ple, cooling of the saltier layer from below across the
boundary of thermal contact causes the cooled saltier fluid
to fall. An intricate pattern of small-scale mixing gradually
develops as moving fluid “fingers” its way downward
(Fig. 4.28). Such double-diffusive instabilities can set up
regular layering in the water column, with layer bound-
aries having high rates of change of temperature and salin-
ity. Double-diffusion in crystallizing magma chambers is
also thought to cause distinct
igneous layering
of different
silicate minerals (Section 5.1).
4.7
Particle settling
4.7.1
A Reynolds number for particles
It is a common occurrence for solid particles to fall
through a still or moving fluid. For example, sand or silt
grains settling out from the atmosphere after a dust storm,
crystals settling through magma, and dead plankton set-
tling through the ocean. In a clearly related phenomenon
of motion, though of opposite sign and somewhat more
complex,
gas bubbles
or immiscible liquid may rise through
other liquids, expanding as they rise through coalescence
and ever-decreasing hydrostatic pressure.
It is reasonable to give a
Re
for solid, liquid, and gaseous
particle motions in fluid (Figs 4.29 and 4.30). A combi-
nation of fluid and solid physical properties defines the
particle Reynolds' number, Re
g
. We use the mean particle
size (diameter,
d
, or radius,
r
d
/2), as the length scale
with which to consider flow interactions. The velocity term
is the relative velocity between particle and fluid,
V
p
.
h
,
fluid molecular viscosity
r
, fluid density
W
p
, ascent velocity
F
viscous
W
rms
, vertical
fluid velocity
s
, particle density
d
, particle diameter
(
W
p
−
w
')(
s
−
r
)d
Re
p
=
h
-
F
, particle weight force
-W
p
, fall velocity
Fig. 4.30
Necessary forces to balance to derive relation for particle
fall velocity.
Fig. 4.29
Necessary parameters to define a particle Reynolds' number.







Search WWH ::

Custom Search