Geoscience Reference
In-Depth Information
CFC molecule
5
Molecule breaks up into
oxygen molecule and chlorine atom,
which is free to react again with
another ozone molecule
Ultraviolet
radiation
Chlorine
atom
1
Oxygen
molecule
Chlorine
atom
CFC molecule absorbs
UV radiation and chlorine
atom breaks away
4
Oxygen atom is pulled
off ClO molecule by
another oxygen atom,
forming another oxygen
molecule
2
CIO
molecule
Chlorine atom reacts
with ozone molecule
CIO
molecule
Ozone
molecule
Oxygen
atom
3
Reaction products are
chlorine oxide (CIO) and
oxygen molecules
Oxygen
molecule
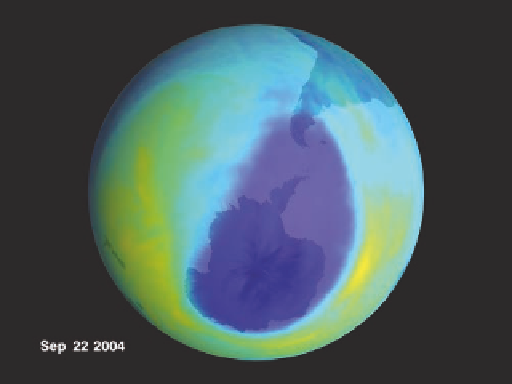
Figure 4.12 Destruction of the ozone layer.
Industrial chlorofluorocarbons (CFCs) interact with UV radiation
in the ozone layer and release chlorine atoms. Follow the numbered steps to see how this process works. It
begins with the breaking away of a chlorine atom from a CFC molecule due to the absorption of UV radiation.
The resultant free chlorine atom then begins to interact with an ozone molecule. The chlorine atoms transform
ozone molecules into ordinary oxygen molecules without being used up themselves. Thus, one CFC molecule
can destroy many ozone molecules.
(a)
(b)
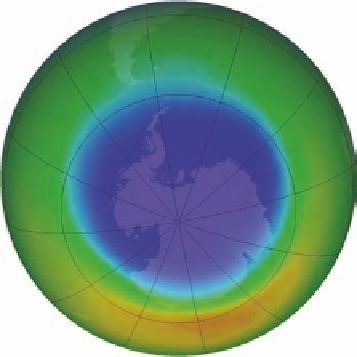
Figure 4.13 Antarctic ozone hole.
(a) Ozone hole during September 2004. The area of the hole was
24,200,000 km
2
(9,400,000 mi
2
), or larger than the combined area of the United States, Canada, and Mexico.
Dark blue represents levels of ozone that are about 20% less than normal. (b) Ozone hole in September 2012.
The average size of the hole in 2012 was 17,900,000 km
2
(6,900,000 mi
2
), making It the second smallest in the
past 20 years.