Geoscience Reference
In-Depth Information
Ultraviolet
radiation
Step 1
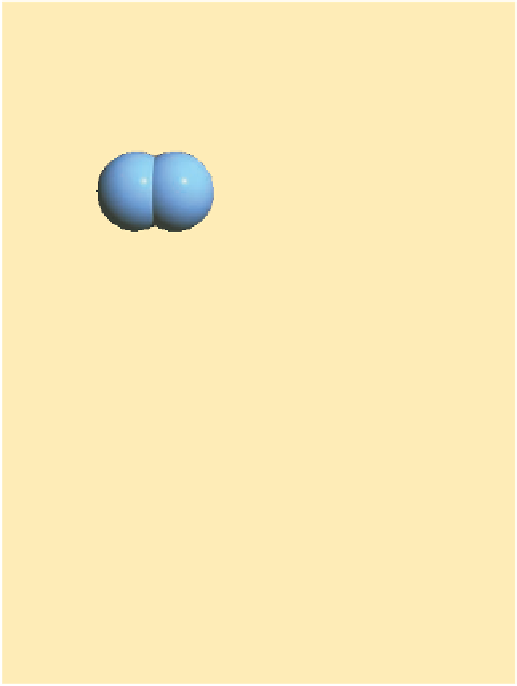
Oxygen
molecule
Oxygen
atom
Oxygen
atom
Figure 4.11 smog in singapore.
A large component of this
haze is ozone created by the interaction of nitrogen oxides
and organic gases emitted by automobiles and factories.
Oxygen
atom
Step 2
layer is critical to life because it absorbs harmful UV radiation
from the Sun that would otherwise reach the Earth's surface.
A major environmental issue that developed in the last half
of the 20th century is the depletion of the ozone layer. This
depletion happened on a global basis due, in part, to the re-
lease of chlorofluorocarbons (CFCs) associated with air con-
ditioners and other cooling systems. CFC molecules are very
stable in the atmosphere and gradually diffuse upward from
the surface to the ozone layer. Once they reach this altitude,
CFCs absorb UV radiation and break down into chlorine ox-
ide (ClO) molecules that, in turn, attack ozone molecules and
convert them into oxygen atoms (Figure 4.12). The net effect
of this process is that the ozone layer is depleted and more
UV radiation reaches the ground because less is absorbed in
the atmosphere.
In the 1980s it was discovered through satellite remote
sensing of the atmosphere that the ozone layer was being
depleted at an alarming rate. Although some depletion occurs
naturally by volcanic aerosols periodically injected into the
atmosphere, most of the reduction was attributed to industrial
CFC production. The most striking example of ozone deple-
tion is the Antarctic
ozone hole,
which was discovered in the
mid-1980s. This feature forms every spring in the Southern
Hemisphere (Figure 4.13), because a polar vortex develops dur-
ing the previous winter that traps the air within it. This vortex is
somewhat analogous to a whirlpool in water that does not allow
a floating object to get out as it spins.
Over the course of each winter, air becomes very cold and
thin clouds of ice form within the atmospheric whirlpool. The
floating ice crystals, in turn, facilitate the transformation of
Ozone
molecule
Oxygen
molecule
Figure 4.9 Formation of atmospheric ozone.
In the first
step, UV radiation is absorbed by oxygen molecules, creat-
ing free oxygen atoms. In the second step, these free oxygen
atoms combine with oxygen molecules to form ozone.
Concentrated Ozone
Smog
Ground-level ozone
Figure 4.10 Ozone concentrations in the atmosphere.
One layer is at high altitudes and absorbs UV radiation from
the Sun. Most of this ozone is concentrated in the ozone
layer. The second concentration occurs in the lower part of
the atmosphere and is associated with pollution, particularly
chemical smog in cities.
Ozone hole
The decrease in stratospheric ozone observed on
a seasonal basis over Antarctica, and to a lesser extent over
the Arctic.