Geoscience Reference
In-Depth Information
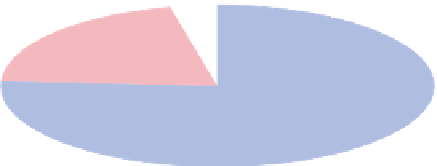
Carbon dioxide
0.037%
Other gases
0.003%
Water vapor
0-4%
Argon
0.93%
Oxygen
20.95%
Nitrogen
78.08%
Figure 4.5 Proportion of various gases in the atmo-
sphere.
Most of the atmosphere consists of nitrogen and
oxygen, which are constant gases. Other gases, such as car-
bon dioxide and water vapor, are variable gases.
Stratosphere
Troposphere
approximately 1% of the atmosphere. It is inert (chemically in-
active) and of little importance in natural processes.
Nitrogen occurs in molecular form as two nitrogen atoms
bonded together (N
2
). This gas makes up 78% of the atmo-
sphere and is derived from the decay and burning of organic
material, volcanic eruptions, and the chemical breakdown
of specific kinds of rocks. Although nitrogen is largely inert
in the atmosphere, it is critical to plant life because it can be
transformed, or fixed, into chemical compounds (ammonia or
nitrates) in the soil. These compounds are absorbed by plants
and incorporated in their tissues as proteins. Nitrogen maintains
a constant proportion of the total atmosphere because what is
added is balanced by what is removed through precipitation and
various biological processes.
Oxygen makes up 21% of the atmosphere and is a by-
product of photosynthesis. In contrast to the inert nature of ni-
trogen, oxygen gas (O
2
) is very active and can combine with
a variety of other elements through the process of oxidation.
Oxygen is essential to animal respiration because it is required
to convert foods into energy. Oxygen is a constant gas because
the amount produced by plants balances the amount absorbed
by various organisms through respiration.
Figure 4.4 The Earth's atmosphere as viewed from
space.
This image nicely demonstrates the thinness of the
atmosphere, which is the faint blue haze that appears on
the horizon. Note the infinite blackness of deep space in the
background.
as a buffer that shields the planet from the potentially harmful
effects of UV radiation from the Sun, allowing mostly vis-
ible and infrared wavelengths to reach Earth. The atmosphere
behaves as a fluid in much the same manner as water, with
flowing currents and eddies. Fluctuations in these currents and
eddies shape the course of environmental conditions on the
Earth's surface at every moment. Knowing how solar radia-
tion flows in the atmosphere is key to understanding Earth's
temperature, atmospheric circulation, and precipitation pat-
terns, which are discussed in later chapters.
In general terms the atmosphere consists of air, an invis-
ible medium that surrounds and protects Earth (Figure 4.4).
The fundamental components of the atmosphere can be divided
into three categories: (1) constant gases, (2) variable gases, and
(3) particulates. Each of these constituents is critical to the way
the atmosphere functions because it performs a unique role
essential to life on Earth.
Variable gases
Variable gases
differ in their proportion of the atmosphere
over time and space, depending on environmental conditions.
Although some of these gases are very important to life, they
make up only a tiny portion (less than 1%) of the atmosphere.
The most important variable gases are carbon dioxide, water
vapor, and ozone.
Constant gases
Constant gases
maintain more or less the same proportion in
the atmosphere. In our atmosphere, nitrogen and oxygen are the
primary constant gases and together make up 99% of the atmo-
sphere (Figure 4.5). Argon is the other constant gas, composing
Constant gases
Atmospheric gases such as nitrogen, oxygen,
and argon that maintain relatively consistent levels in space
and time.
Variable gases
Atmospheric gases such as carbon dioxide,
water vapor, and ozone that vary in concentration in space and
time.