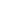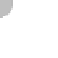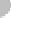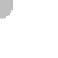Geoscience Reference
In-Depth Information
NEUTRAL
More acidic
More acidic
More basic
More basic
pH
0
0
1
1
2
2
3
3
4
4
5
5
6
6
7
7
8
8
9
9
10
10
11
11
12
12
13
13
14
14
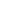
Household
ammonia
(11.0-12.0)
Stomach acid
(1.0-3.0)
Beer (4.0-4.5)
Banana (4.8)
Milk (6.4)
Blood (7.4)
Soaps,
detergents
(10.0)
Milk of
magnesia
(10.5)
Lye (13.0)
Lemon juice
(2.2-2.4)
Apple (3.2)
Rainwater (5.6)
Vinegar
(2.4-3.4)
Figure 11.17 The pH scale.
pH refers to the concentration of hydrogen ions (H
+
) in solution. Note that most natural foods are slightly
acidic and most common cleaning agents are basic.
from 0 to 14, with lower values indicating acidic conditions and
higher values indicating basic (alkaline) conditions. Neutral pH
is about 7, which is the pH of pure water. To get a feel for pH
and how it relates to various common products, see Figure 11.17.
In the context of soils, pH is an important indicator of soil fertil-
ity. Highly alkaline soils, for example, are not efficient with respect
to dissolving minerals and making them available as nutrients for
plants. Acidic soils, on the other hand, result in extensive leach-
ing of minerals—so much so that many nutrients are completely
lost before they can ever be consumed by plants. In general, most
plants, including agricultural crops, are adapted to soils that have a
neutral or near-neutral pH. It is possible, however, to treat slightly
acidic soils with alkaline fertilizers, such as slaked lime (calcium
carbonate, CaCO
3
), to raise the pH to the appropriate level.
ions through the process of cation exchange. Soils that have a
high
cation exchange capacity
(CEC)
are typically the most
fertile because they contain an abundance of colloids on which
cations can be held and exchanged.
In the context of soil fertility, colloids are important
because they keep minerals from being completely leached
from the soil. Often, cations are held by the colloid until a plant
root comes into contact with the colloid and absorbs the cation
as a nutrient. Figure 11.18 illustrates how individual negatively
charged colloid particles (in this case, clays) attract the posi-
tively charged cations surrounding them until a plant root “con-
sumes” the stored nutrients.
Colloids and Cation Exchange
An important part of soil chemistry is the concept of
colloids
,
which are extremely small (
<
0.001 millimeter) particles that
exist in inorganic and organic form. Inorganic colloids consist of
crystalline clays that are thin and platy. These microscopic bodies
are created when larger particles are chemically altered through
weathering. Organic colloids, in contrast, consist of humus
derived from organic matter. Regardless of whether colloids are
inorganic or organic, they are critical to soil chemistry because
they are chemically active and have a high water-holding capacity.
Colloids are also important to soil chemistry because they
hold and exchange cations.
Cations
are positively charged
(atomic) ions that exist in soil solution due to the dissolution
of soil minerals, such as calcium, magnesium, iron, and potas-
sium. Because soil colloids are negatively charged, they attract
the positively charged cations that are suspended in the soil
solution. Although colloid particles are individually small, they
collectively have a very large surface area on which cations can
become attached. Some ions, such as metal and hydrogen ions,
are bound tightly to colloids, whereas basic ions such as calci-
um move with relative freedom. In many instances, the loosely
bound (basic) cations are replaced by the metallic and hydrogen
Mg
2
+
ion
K
+
ion
Ca
2
+
ion
Water
Plant root
Colloid particle
Figure 11.18 Microscopic view of colloids and soil fertility.
Col-
loids are microscopic clays and organic particles that are negatively
charged. Cations, which are positively charged atoms that exist in
soil solution, are thus attracted to the colloid particles, which hold
them until a plant root absorbs them.
Colloids
Very small (10 nanometers to 1 micrometer), evenly
divided solids that do not settle in solution.
Cation exchange capacity (CEC)
The total amount of
exchangeable cations that a soil can absorb.
Cations
Positively charged ions, such as sodium, potassium,
calcium, and magnesium.