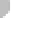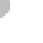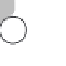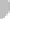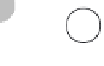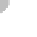Geoscience Reference
In-Depth Information
Physical Properties of Water
more strongly than do the hydrogen atoms. Because of this unequal
attraction, the oxygen end of the molecule has a partial negative
charge, whereas the hydrogen side has a partial positive charge.
Water molecules are attracted to each other because of
these contrasting positive and negative charges. The negative
end of one molecule is attracted toward the positive end of an
adjacent molecule. This is why water molecules stick together
in a drop of water and why water occurs in liquid form at normal
surface temperatures. (Most chemical compounds are solids or
gases at normal surface temperatures.) The attraction between
molecules of water is known as
hydrogen bonding
because hy-
drogen atoms form particularly good bonds between molecules.
These bonds are strongest, of course, when water is frozen as
ice. In these circumstances, the molecules firmly bond to each
other in distinct hexagonal forms (Figure 7.1b).
Another interesting attribute of water is its high surface ten-
sion. Surface tension results when molecules at the surface of a
liquid have a strong attachment to each other but not to the mol-
ecules of air above them. This attraction is particularly strong in
water because of hydrogen bonding. Thus, the water molecules
pull harder to the sides to create the smallest possible amount of
surface area, forming spherical bubbles and droplets. The high
surface tension also creates an elastic “skin” on the surface of wa-
ter, strong enough to allow some kinds of insects (such as water
striders) and even small lizards to walk on the surface.
Yet another important feature of water is its ability to move
upward in thin openings (or capillaries) against the force of
gravity within the soil and plants in a process called
capillary
action
. This motion occurs because water molecules pull other
water molecules along through hydrogen bonding. This process
is very important because it enables plants to transport nutrients
from their roots up into their stems and leaves.
Have you ever really thought about the character of water, the
way it feels, flows, and freezes? Water is fascinating stuff if you
really stop to think about it. It is found almost everywhere on
Earth and is crucial to life as we know it. Water covers 71% of
our planet's surface and even comprises about 70% of the human
body by weight. It is a very important component of the atmo-
sphere because it stores energy that contributes to global wind
patterns. In addition, it plays a major role in the regulation of
climate. A good place to start investigating how water behaves in
the physical environment is by first reviewing its basic properties.
Hydrogen Bonding
The logical place to begin a discussion of water is the way that
water molecules are bound to each other. A drop of water is made
up of billions of molecules, with each consisting of two hydrogen
atoms combined with one oxygen atom (Figure 7.1a). This combi-
nation is the basis for water's well-known chemical formula: H
2
O.
Within a water molecule, oxygen attracts the bonding electrons
O
-
H
+
H
+
O
-
Hydrogen bonds
between unlike
charges
-
H
+
H
+
O
-
H
+
-
O
(a) Water Molecules
Thermal Properties of Water
and Its Physical States
One of the most important characteristics of water is that it ab-
sorbs and releases latent heat, which, if you recall from Chapter 4,
is hidden energy stored in molecular bonds. This form of hidden
energy, which cannot be detected, contrasts with sensible heat that
is felt (or “sensed”). The capability of water to store and release
latent heat is important because it contributes significantly to at-
mospheric circulation and helps regulate climate. Understanding
this property is also important because it explains how water can
exist in the three physical states, or phases, familiar to you: solid
(as
ice
), liquid (as
water
), and gas (as
water vapor
). The movement
of water from one phase to another occurs when hydrogen bonds
are formed, loosened, broken entirely, or tightened.
The transformation of these bonds is directly related to the
ways that heat energy interacts with water molecules. A simple
rule of thumb is that heat energy must be applied to molecules if
hydrogen bonds are to be loosened or broken, and extracted from
(b) Ice
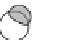
Figure 7.1 Chemical composition of water.
(a) Water mol-
ecules are composed of two hydrogen atoms and one oxygen
atom, which form partially positive ends of molecules (hydrogen)
and partially negative ends (oxygen). Given the attraction between
positive and negative charges, relatively weak hydrogen bonds
are produced between molecules. (b) Hydrogen bonds are the
reason liquid water molecules form solid ice at a comparatively
high temperature; it takes more energy to break these bonds than
is the case for most other common liquids.
Capillary action
The process through which water is able to
move upward against the force of gravity.