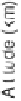Geoscience Reference
In-Depth Information
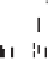
Fig. 2. Comparison of CO
2
production and loss rates calculated from several photo-
chemical models. The solid line is the CO
2
photolysis rate from the nominal chemistry
model. The dashed-triple-dotted line shows production of CO
2
via Reaction (1) from
the O
2
(c) model. The long-dashed line shows production of CO
2
via ClCO
3
from the
P2004 model. The dotted line shows production of CO
2
via Reaction (2) from the aerosol
chemistry model.
5. Conclusions and Recommendations
Either chlorine or aerosol catalytic chemistry may be able to explain the
low-O
2
abundance on Venus. The chlorine catalytic mechanism
6
has been
validated
4
but the rates for the component reactions are poorly constrained.
An aerosol catalyzed mechanism to produce CO
2
has not been demon-
strated conclusively yet, so the rate(s) are unknown.
A multi-dimensional chemical transport model is needed and
Venus
Express
will provide the information needed to guide development of such
a model. Wind fields, temperatures, and the distribution of CO on the day
and night sides at 60-100 km from
Venus Express
will be particularly useful
for stepping up to a multi-dimensional chemical transport model.
Laboratory work is required to interpret data from
Venus Express
and
improve the quality of photochemical modeling. Tighter constraints on the
temperature dependent rate for ClCO + O
2
+
M
ClCO
3
+
M
and the
temperature dependent equilibrum constant for ClCO are needed. The e-
cacy of aerosol catalytic mechanism(s) for production of CO
2
needs to be
quantitatively evaluated, and the rate for Reaction (1) needs to be deter-
mined along with the rates of competing loss reactions for O
2
(c
1
Σ).
Several sets of observations are needed and many are possible from
Venus Express
. The abundance of ground-state O
2
; coincident observations
of the distributions of CO and O
2
(a
1
∆) airglow; simultaneous retrievals of
CO distributions, winds, and temperatures; and profiles for O
3
and OCS
→