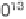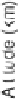Geoscience Reference
In-Depth Information
Fig. 1. Comparison of O
2
concentrations calculated from several photochemical models
and the O
2
profile used to interpret the spectroscopic upper limit. The dotted line shows
the results from the nominal chemistry model. The dashed-triple-dotted line is from the
O
2
(c) model. The short-dashed line is from the P2004 model. The solid line is from
the aerosol chemistry model. The long-dashed line is the O
2
profile used to interpret
the spectroscopic upper limit observation.
5
90%.
11
The rate for loss of O
2
(c
1
Σ) due to collisions with CO
2
was assumed
to be 1
×
10
−
15
cm
3
/s,
16
and the rate for Reaction (1) was assumed to be
1
10
−
11
cm
3
/s. The rate for Reaction (1) has not been measured. The
sensitivity of the model calculations to these parameters will be explored
in a forthcoming publication.
The P2004 model
4
included several adjustments to enhance the
effectiveness of the gas-phase catalytic chlorine chemistry.
6
The thermal
dissociation rate for ClCO was decreased by 1.5 times the recommended
uncertainty in the ClCO equilibrium constant,
25
the rate for ClCO + O
2
+
M
×
ClCO
3
+
M
was increased slightly, and temperatures at 84-96 km
altitude were decreased by 6 K. The sensitivity of the model calculations to
the thermal stability of ClCO is illustrated by the difference between the
P2004 and the nominal chemistry models in Fig. 1.
The aerosol model examined the effect of incorporating heterogeneous
chemistry, Reaction (2), into a model that otherwise included only gas-
phase chemistry. Reaction (2) was added to the P2004 model with a reactive
uptake coecient,
γ
,of10
−
5
, which is believed to be a plausible rate (L.
Phillips, personal communication, Jan 2005). At this rate, CO
2
production
via Reaction (2) was comparable to that via the gas-phase chlorine catalytic
pathways. The mixing ratio of OCS at the lower boundary was increased to
10 ppb
31
to better match the cloud level CO observation.
32
If
γ
is increased
to 10
−
4
, then the calculated O
2
column abundance decreases by almost
afactoroftwo.If
γ
is less than about 3
→
10
−
6
, then the Cl chemistry
dominates production of CO
2
, as it does in the P2004 model.
×