Geoscience Reference
In-Depth Information
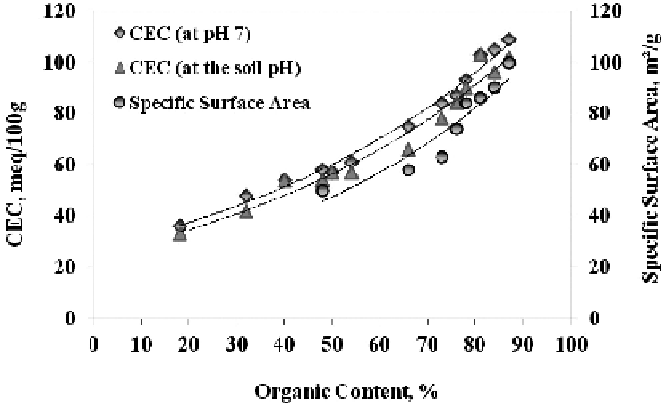
Figure 3.11
Organic content - CEC (
after
Asadi
et al
., 2009d).
2mM BaCl
2
-2H
2
O and centrifuging is repeated twice. Before the third centrifuging
the pH of the slurry is obtained. After decanting 2mM BaCl
2
-2H
2
O, 10mL of 5mM
MgSO
4
is added and shaken gently for one hour. The conductivity of the 1.5mM
MgSO
4
is determined. If the conductivity of the sample is not 1.5 times this value,
0.1mL increments of 0.1M MgSO
4
are added until it is. The pH of the solution is
determined. If it is not within 0.1 units of the previous measurement, 0.05M H
2
SO
4
is added dropwise until the pH is in the appropriate range. Finally, distilled water is
added until the solution's conductivity is that of 1.5mMMgSO
4
and the tube is dried
and weighed. The CEC is calculated based on the total solution, Mg in solution and
the total Mg added, i.e. CEC (meq/100g) is equal to the total Mg (meq) added minus
Mg (meq) in the final solution.
Figure 3.11 shows the cation exchange capacity of various organic soils.
The CEC of organic and peaty soils generally increases with increasing organic con-
tent. The water adsorption potential of organic soils generally increases with increasing
CEC (Asadi
et al
., 2009d).
Figure 3.12 shows the cation exchange capacity of various organic soils according
to the degree of humification. The CEC of organic and peaty soils increases with
increasing degree of humification (Asadi
et al
., 2009d).
Both clay and organic materials can contribute to the CEC of organic soils. Because
cations are positively charged, they are attracted to surfaces that are negatively charged.
Although there is a qualitative difference between the clay and organic matter fractions
of organic soil, the role of the clay fraction is also very important for providing cation
exchange sites.
Since the charge in organic soils is strongly pH dependent, the resulting soils
develop a greater CEC at near-neutral pH than under acidic conditions. Therefore
the main problem with measurement of CEC at pH 7 is that it buffers the soil at