Geoscience Reference
In-Depth Information
Step 1
Diusion to adsorbent surface
Step 2
Migration into pores of adsorbent
Step 3
Monolayer buildup of adsorbate
Contaminant
molecules
Contaminant
molecules
Contaminant
molecules
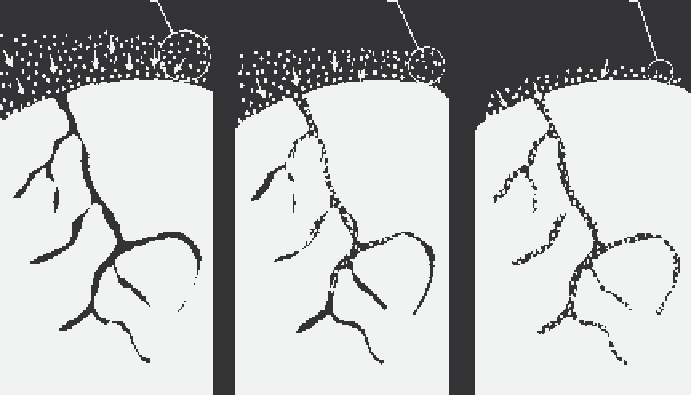
FIGURE 16.12
Gas collection by adsorption. (Adapted from USEPA,
APTI Course 415: Control of Gaseous
Emissions
, EPA 450/2-81-005, U.S. Environmental Protection Agency Air Pollution Training Institute,
Washington, DC, 1981, p. 5-2.)
The efficiency of most adsorbers is near 100% at the beginning of the operation and remains
high until a breakpoint or breakthrough occurs. When the adsorbent becomes saturated with adsor-
bate, contaminant begins to leak out of the bed, signaling that the adsorber should be renewed or
regenerated. Although adsorption systems are high-efficiency devices that may allow recovery of
product, have excellent control and response to process changes, and have the capability of being
operated unattended, they also have some disadvantages, including the need for expensive extrac-
tion schemes if product recovery is required, relatively high capital cost, and gas stream prefiltering
needs (to remove any particulate capable of plugging the adsorbent bed)(Spellman, 2008).
16.4.1 a
dsorption
s
teps
Adsorption occurs in a series of three steps (USEPA, 1981, p. 5-2). In the first step, the contaminant
diffuses from the major body of the air stream to the external surface of the adsorbent particle. In
the second step, the contaminant molecule migrates from the relatively small area of the external
surface (a few square meters per gram) to the pores within each adsorbent particle. The bulk of
adsorption occurs in these pores because the majority of available surface area is there (hundreds of
square meters per gram). In the third step, the contaminant molecule adheres to the surface in the
pore. Figure 16.12 illustrates this overall diffusion and adsorption process.
16.4.2 a
dsorption
F
orCes
—p
hysiCal
and
C
hemiCal
The adsorption process is classified as either
physical
or
chemical
. The basic difference between phys-
ical and chemical adsorption is the manner in which the gas molecule is bonded to the adsorbent. In
physical adsorption, the gas molecule is bonded to the solid surface by weak forces of intermolecular
cohesion. The chemical nature of the adsorbed gas remains unchanged. Therefore, physical adsorption
is a readily reversible process. In chemical adsorption, a much stronger bond is formed between the
gas molecule and adsorbent. A sharing or exchange of electrons takes place. Chemical adsorption is
not easily reversible.
Search WWH ::

Custom Search