Geoscience Reference
In-Depth Information
Explicit scheme,
α
= 1
150
100
analytical solution
time step 0.1
time step 0.5
time step 1.0
time step 1.5
time step 2.1
50
0
0
2
4
6
8
10
12
−
50
−
100
−
150
time
FIGURE 6.5
Solution of the decay equation (Equation (6.13)) with the explicit scheme with
different time steps.
As long as
∆
t
is small (more precisely
α∆
t
<
1
), the solution is approximating
the exponential decay. But once
∆
t
becomes equal to
1/
α
, the solution reads:
t
+
∆
t
t
C
=
()
0
C
=
0
i
i
and so, in the first time step, the value of the concentration drops to 0 and then stays
there. Even worse, if
∆
t
>
(/ )
1
α
then
(
1
−
α∆
t
)
<
0
and concentrations become nega-
tive, a completely nonphysical behavior.
However, even with these negative values, the solution of the decay equation is
still stable because the oscillations generated are slowly decaying. However, if has
been chosen to be , then , and the oscillations start to
amplify instead of decaying. There is no mechanism to dampen these oscillations
and so they will amplify to reach arbitrary large (positive and negative) values. The
solution has become unstable.
This behavior is shown in Figure 6.5 where the solution to the decay equation
with has been plotted. As can be seen, all solutions with a time step of less
than 1 are stable and are not undershooting. The solution with drops to 0 in
the first time step, whereas for the solution produces negative values, but
the solution is still stable. Finally, for the solution becomes unstable.
The situation changes completely when the implicit approach is used. Now the
discretized equation reads
∆
t
∆
t
>
(/ )
2
α
(
1
−
α∆
t
)
< −
1
α =
1
∆
t
=
1
∆
t
=
1.
∆
t
=
2.
t
+
∆
t
t
t
+
∆
t
CC tC
=−
α
∆
or, after solving for the concentration on the new time level,
1
t
+
∆
t
t
C
=
t
C
1
+
α
∆























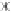

















































Search WWH ::

Custom Search