Geoscience Reference
In-Depth Information
Air
Water
Air
Water
Microorganisms
(1)
ROO
′
(2)
A
B
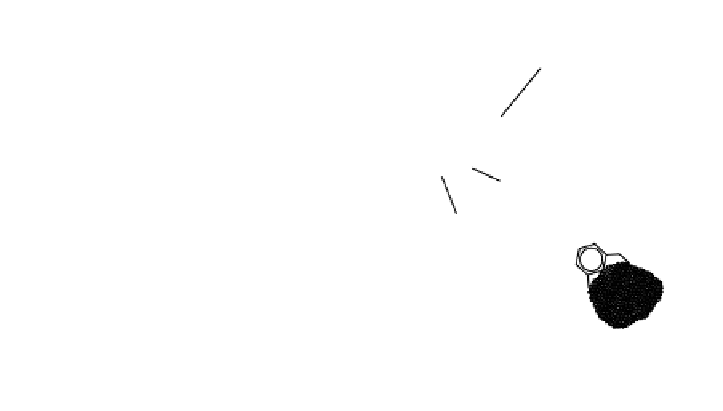
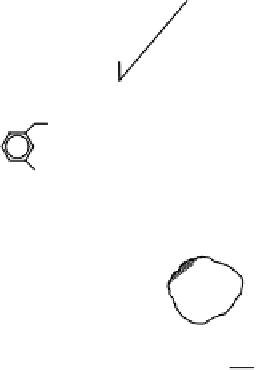
FIGURE 4.8
Some processes in which sorbed species behave differently than dissolved
molecules of the same substance. (A) Dissolved species can volatilize (1), whereas sorbed
species can settle to the sediment (2). (B) Dissolved and sorbed species react at different rates.
Sorption is extremely important, because it may dramatically affect the fate and
impact of a chemical in the aquatic environment. Structurally identical molecules
behave very differently if they are surrounded by water molecules and ions as
opposed to adsorbing onto the exterior of solids or being absorbed within a solid
matrix.
106
As illustrated in Figure 4.8, sorption can cause a compound to accumulate
in bed sediment or bioconcentrate in fish. It can retard processes such as volatilization
and base hydrolysis, or enhance others such as photolysis and acid-catalyzed hydrol-
ysis.
78
The sorption of toxicants to suspended particulates and bed sediment is a
significant transfer mechanism. Partitioning of a chemical between particulate matter
and the dissolved phase is not a transformation pathway; it only relates its concen-
tration in the dissolved phase to that in the solid phase.
45
Figure 4.8A is an example
of dissolved species that can volatilize while sorbed species may settle to sediment,
whereas Figure 4.8B presents dissolved and sorbed species that react at different
rates.
A significant portion of the impurities in water is found in the suspended matter,
where the concentration can be much higher than in the water. Transport of pollutants
in aquatic systems often takes place on suspended matter, either clay particles or
organic matter (cohesive particles). This implies that many pollutants, otherwise
adsorbed or fixed by ion exchange on the sediment and, therefore, not transported
by clear water, are transported to a greater distance by water with high turbidity (see
Section 4.2.4).
For neutral organics, several mechanisms are involved in the sorption process.
These include hydrophobic effects that cause the sorbate to associate with organic
matter in the particulate phase because of an unfavorable free-energy cost of staying
























Search WWH ::

Custom Search