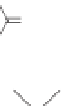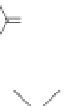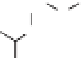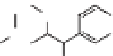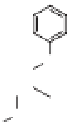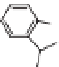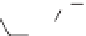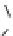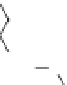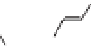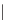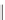Geoscience Reference
In-Depth Information
CH
3
CH
3
CH
3
CH
2
OH
CH
2
OH
CH
2
OH
NH
NH
NH
O
O
O
H
H
H
H
H
H
H
H
H
OH
OH
OH
OH
H
OH
H
OH
H
O
O
O
O
O
O
H
H
H
NH
NH
NH
CH
2
OH
CH
2
OH
CH
2
OH
CH
3
CH
3
CH
3
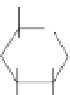
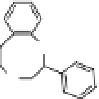
Fig. 4. Example structure of chitin.
fossil cuticles release series of alkanes and alkenes
upon pyrolysis, suggesting that the chitin has been
replaced and/or transformed by an aliphatic geopo-
lymer. Experimental evidence suggests that these
aliphatic compounds may in fact be lipids that
have become attached to the biomacromolecule.
These lipids are likely to originate from the closest
source available, the organism itself (Gupta et al.
2006, 2007b; de Leeuw 2007).
via the phenylpropanoid pathway, namely the mon-
olignols p-coumaryl, coniferyl and sinapyl alcohols
(XXXXI-XXXXIII) (de Leeuw & Largeau 1993;
Raven 2000) (Fig. 4). The polymerization reaction
has long been considered to be a random process
but this concept appears to be wrong (see reviews
of both Lewis 1999; Davin & Lewis 2005). The
corresponding degradation products are coumaryl,
guaiacyl
(or vanillyl)
and syringyl moieties
(XXXXIV-XXXXVI),
respectively (Hedges &
Aromatics and their polymers - lignin. Aromatic
polymers are lignin (Fig. 5) as are at least some spor-
opollenins (Boom 2004). There are some enigmatic
algal biomacromolecules with high preservation
potential such as the wall material of dinoflagellate
cysts. The wall material is currently referred to by
the cryptic name 'dinosporin'. Although dinosporin
has been suggested to be aromatic with the isopre-
noid tocopherol as an aromatic building block
(Kokinos et al. 1998), this view has been challenged
by others (de Leeuw et al. 2006). The position of
dinosporin in the scheme presented above therefore
remains unclear. Apart from this single report of a
possible aromatic signature in dinosporin and the
increase of phenolic moieties in the algaenan of
the Ordovician freshwater acritarch Gloeocapsa-
morpha prisca in relation to salinity increase
(Derenne et al. 1992), the presence of aromatic moi-
eties seems to be a feature of terrestrial biomacro-
molecular organic matter.
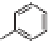
Lignin is a macromolecule resulting from the
polymerization of three phenolic units synthesized
Mann 1979).
Differences in the abundance of the structural
lignin compounds are observed among higher
plants: guaiacyl units dominate in gymnosperms
wood, syringyl and guiacyl units are dominant
in woody tissues of dicotyledonous angiosperms,
p-coumaryl and guaiacyl dominate in woody tissues
of monocotiledonous angiosperms and non-woody
tissues are generally dominated by p-coumaryl
units (Hedges & Mann 1979; Logan & Thomas
1985). Pteridophyte lignins are derived from
sinapyl alcohol (Barcelo et al. 2007).
From these observations, the respective abunda-
nce of the three units in sedimentary organic matter
should change in parallel with the evolution of land
plants (Logan & Thomas 1987). Although structural
motifs of syringyl peroxidases (an enzyme in lignin
synthesis) have been identified in Bryophytes (Ros
et al. 2007), lignin is absent in these plants (Lewis
& Yamamoto 1990). Lignin has also been reported
from a red algae which is considered a case of par-
allel evolution of lignin synthesis (Martone et al.
OH
OH
O
O
OH
OH
H
O
H
H
O
OH
O
O
OH
H
H
O
O
H
O
H
H
O
O
OH
O
OH
OH
O
O
O
O
O
O
O
O
O
OH
H
OH
O
H
O
OH
O
O
OH
OH
Fig. 5. Example structure of lignin (based on Holtman et al. 2003).