Geoscience Reference
In-Depth Information
Solid
Phase
Gas
Phase
Liquid
Phase
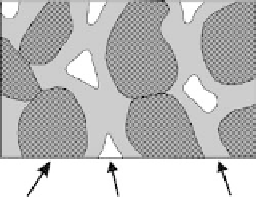
fIGURe 5.2
M
agniied thin section of a soil. Solid-phase quartz particles are good electric current insula-
tors, and clay mineral and organic matter particles transmit current if surfaces are wetted. The gas phase is
essentially made up of air, which does not transmit electric current. The soil solution liquid phase contains
dissolved ions and therefore has the capability to deliver electric current.
particle size into sand (2.0 to 0.05 mm), silt (0.05 to 0.002 mm), and clay (less than 0.002 mm) frac-
tions. Quartz, considered an excellent electric insulator, usually dominates a soil's sand and silt size
fractions. The clay size fraction is made up primarily of clay minerals and organic matter. Given
sufficiently wet conditions, clay minerals and organic matter contribute significantly to electric cur-
rent flow in soil, and more information on this topic will be provided later in the section. The soil
gas phase is mostly air, which is a good insulator, and like quartz, will oppose the flow of electric
current. The soil liquid phase is an electrolytic aqueous solution, referred to as the “soil solution.”
An electrolyte is a chemical substance that will dissociate into ions within a solution. There are usu-
ally a variety of dissolved anions and cations in the soil solution, and some of the most common are
SO
4
2−
, Cl
−
, HCO
3
−
, NO
3
−
, PO
4
3−
, Ca
2+
, Mg
2+
, K
+
, Na
+
, and NH
4
+
.
Unlike a copper wire, where the electric current charge carriers are electrons, dissolved ions
within the soil solution serve as the electric current charge carriers in a soil. Therefore, the electric
current in soil is largely electrolytic, meaning that the flow of electric current is governed sub-
stantially by the movement of dissolved ions in the soil solution. Insight regarding the resistivity
behavior of sandy and silty soils is provided by Archie's law, which is based on electrolytic current
flow through a porous media. Archie's law is empirical, and it quantifies the relationship between
the overall porous media resistivity, the resistivity of the electrolytic aqueous solution present in the
porous media, and the amount of electrolytic solution present per unit volume of porous media. The
law was developed particularly for clay-free rocks and sediment, and therefore, can also be used
for soils that contain essentially no clay minerals or organic matter. The form of Archie's law most
applicable to both saturated and unsaturated conditions for a sandy or silty soil is given as follows:
ρ
W
ρ
=
z
23
(5.3)
1
z
z
ϕ
S
where ρ is the overall soil resistivity; ρ
W
is the resistivity of the soil solution; φ is the porosity (vol-
ume fraction of soil not part of solid phase);
S
is the saturation (volume fraction of the porosity filled
with soil solution);
z
1
is a constant with a value between 0.5 and 2.5, often initially approximated by
1.0;
z
2
is a constant ranging from 1.3 to 2.5, but closer to 1.3 for loose sediments; and
z
3
is a constant
with a value usually close to 2 when
S
is greater than 0.3 (Keller and Frischknecht, 1966; Parasnis,
1986; Reynolds, 1997). Inspection of Equation (5.3) shows that ρ for a sandy or silty soil depends on
ρ
W
and the volumetric water content, θ, which can be defined as the volume of soil solution per unit
volume of soil, equaling the product of φ and
S
(θ = φ
S
).
It warrants pointing out that the values of ρ
W
and θ are often, but not always, interrelated. One
clear example is where higher-temperature conditions lead to evapotranspiration-associated losses