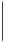
Geoscience Reference
In-Depth Information
(a) Consumption of chemical species
NO
3
−
SO
4
2
−
O
2
0
100%
SO
4
2
−
NO
3
−
O
2
Ox
SWI
SR
Rapid reduction of
nitrate
Sediments act as a
sink for O
2
, SO
4
2
−
and
NO
3
−
Low sulphate
concentrations in
freshwater
If water body deep
and unmixed, water
column anoxia may develop
(b) Production of chemical species
NH
4
+
PO
4
2
−
Mn(II)
Metals
(aq)
CH
4
Fe(II)
NH
4
+
CH
4
Me
Ox
SWI
Phosphate released
from organic matter
breakdown
SR
Methane gas
produced by
methanogenesis
Fe levels
buffered by
presence of
sulfide
FeR + MnR
Precipitation
of vivianite
Phosphate buffered
by vivianite
precipitation
Precipitation
of siderite
(if bicarbonate
activity high
enough)
Meth
Sediments act as a
source of metals, nutrients and methane
Metals adsorbed
onto iron oxides
released during FeR
NH
4
+
released
during organic matter
oxidation
Fig. 6.11
Idealized summary of the results of early diagenetic reactions for sediment and porewaters in freshwater urban water bodies (docks, canals and lakes). Typical values and depths
derived from data published in Taylor et al. (2003) and Dodd et al. (2003). Porewater profiles are idealized and will vary with variations in sediment composition and accumulation rate: Ox,
aerobic oxidation; SR, sulphate reduction; FeR, iron reduction; MnR, manganese reduction; Meth, methanogensis; SWI, sediment-water interface.