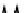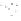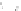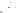Geoscience Reference
In-Depth Information
(a) Low flow conditions
Road surface
Water and sediment input
Water and fine sediment
Coarser
sediment
settles out
Water
Sediment
(b) Wet weather conditions (high flow)
Water and sediment input
Water and sediment
Resuspension
(c) Dry weather conditions (no flow)
PO
4
2
O
2
−
NH
4
+
Oxygen consumed
by sediment
CH
4
Chemical species
released from sediment
into liquor
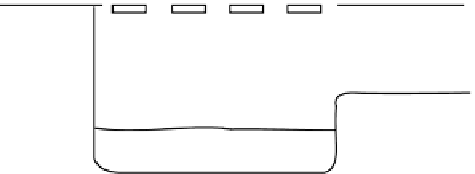
Fig. 6.8
Schematic diagram of the
sediment processes operating within a
gully pot.
at the kerbside. They are a major feature of urban
drainage networks, with more than 17 million
present in the UK alone (Memon & Butler 2002a).
The trapping of this sediment is desirable for
two reasons. First, it minimizes the amount of
sediment that enters into the sewerage system
and, thereby, reduces the problems caused by
sediment accumulation in sewers. Second, where
gully pots are emptied frequently, it minimizes
the amount of sediment that is potentially flushed
out of the sewer system into rivers and receiving
water bodies. The design and assessment of gully
pots has been undertaken primarily in the field
of civil engineering, where the term
sludge
is used
to describe the sediment in a pot and
liquor
to
refer to the in-place water.
The processes acting within gully pots are
complex. During runoff events (wet weather
processes), denser particles in the water will
settle out under gravity (Fig. 6.8). However, there
is usually a high degree of turbulence within
the gully pot, which not only limits the amount
of sediment that will settle out, but may also
lead to the erosion and re-suspension of existing
sediment in the pot. As well as the physical pro-
cesses taking place, major biochemical changes
can take place within the gully pot (Fig. 6.8).
Most biochemical changes take place during