Geoscience Reference
In-Depth Information
Table 4.4
A geochemical classification (Berner 1981) of sedimentary environments.
C
is the concentration (moles L
−
1
).
H
2
S is total sulphide.
Environment
Characteristic phases
10
−6
)
II. Anoxic (CO
2
<
I. Oxic (CO
2
≥
Haematite, goethite, MnO
2
-type minerals; no organic matter
10
−6
)
A. Sulphidic (CH
2
S
10
−6
)
B. Non-sulphidic (CH
2
S
≥
Pyrite, marcasite, rhodochrosite, alabandite; organic matter
Glauconite and other Fe
2+
-Fe
3+
silicates (also siderite, vivianite, rhodochrosite);
no sulphide minerals; minor organic matter
Siderite, vivianite, rhodochrosite; earlier formed sulphide minerals;
organic matter
10
−6
)
<
1. Post-oxic
2. Methanic
important phase for direct biological uptake.
Operationally, the limit between the particulate
phase and the dissolved phase is generally deter-
mined by means of filtration, using a pore size
of 0.45
approach, and many colloidal particles will pass
through such filters.
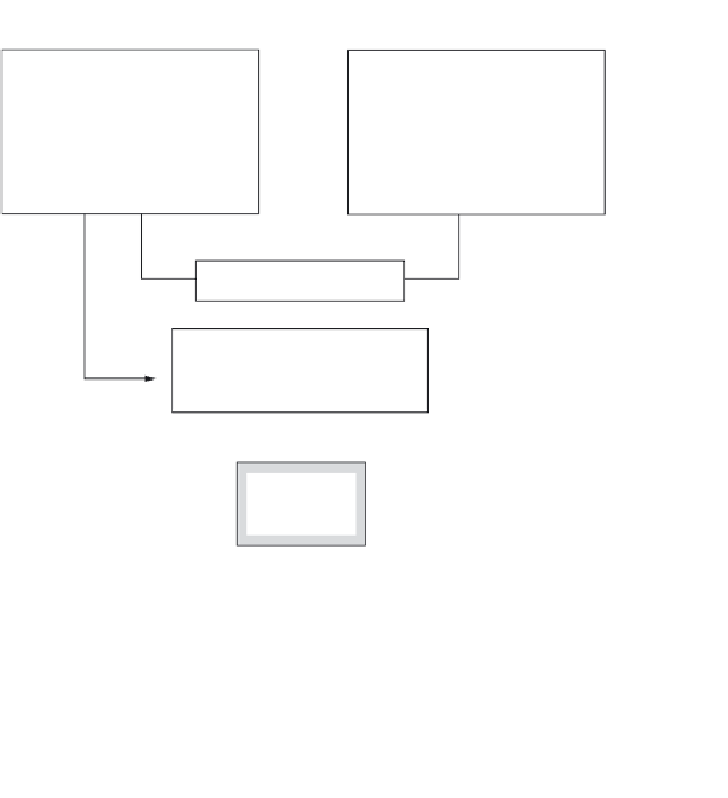
A general outline of particles in lake water,
their origin, standard abbreviations and a classi-
fication scheme are given in Fig. 4.4. As stressed,
μ
m. Evidently, this is an operational
Particles in lake water
Allochthonous material
Autochthonous material
- Minerogenic particles (clays,
silt, sand, Fe-Mn oxides and
hydroxides, etc.)
- Organic particles (humic
matter, living and dead plankton,
etc.)
- Organic particles (living and
dead plankton, etc.)
- Inorganic matter (e.g. ashes,
shells)
Origin,
primary
Resuspended particles
Origin,
secondary
Suspended particulate matter
(SPM = seston)
- Dead versus living
- Inorganic versus organic
Particulate
inorganic
matter (PIM)
Total organic
matter (TOM)
100%
Particulate
organic matter
(POM)
20%
Dissolved
organic matter
(DOM)
80%
Fig. 4.4
Classification
scheme and
nomenclature for
particles in lake water
(see also Dubko 1985;
Ostapenia 1985).
Conservative
organic
matter
68%
Reactive
organic
matter
12%
Plankton
4%
Detritus
16%