Geoscience Reference
In-Depth Information
should then be left at 20°C for five days in a dark-
ened environment. After this the dissolved oxygen
content should be measured again. The difference
between the two dissolved oxygen readings is the
BOD
5
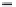
value. Over an extended period the dissolved
oxygen content of a polluted water sample will
look something like that shown in Figure 7.4. In
this case the dissolved oxygen content has dropped
from 9.0 on day one to 3.6 on day five, giving a
BOD
5
value of 5.4 mg/l. After a long period of time
(normally more than five days) oxygen will start to
be consumed by nitrifying bacteria. In this case the
bacteria will be consuming oxygen to turn nitro-
genous compounds (e.g. ammonium ions) into
nitrate. In order to be sure that nitrifying bacteria
are not adding to the oxygen demand a suppressant
(commonly allyl thiourea or ATU) is added. This
ensures that all the oxygen demand is from the
decomposition of organic matter. The use of a five-
day period is another safeguard, as, due to the
slow growth of nitrifying bacteria, their effect is
not noticeable until between eight and ten days
(Tebbutt, 1993). There is an argument to be made
saying that it does not matter which bacteria are
causing the oxygen demand, the test should be
looking at all oxygen demand over a five-day period
and therefore there is no need to add ATU. However
the standard BOD test uses ATU to suppress the
nitrifying bacteria.
15
13
11
9
7
5
0
10
20
30
Temperature (
°
C)
Figure 7.3
Relationship between maximum dissolved
oxygen content (i.e. saturation) and temperature.
whereas coarse fish (e.g. perch, pike) can survive in
levels as low as 2 mg/l. The dissolved oxygen con-
tent is also an important factor in the way we taste
water. Water saturated in oxygen tastes fresh to
human palates; hence drinking water is almost
always oxygenated before being sent through a pipe
network to consumers.
There are two methods by which dissolved
oxygen content is considered: percentage saturation
and concentration (mg/l). These two measures are
interrelated through temperature, as the dissolved
oxygen content of water is highly temperature
dependent (see Figure 7.3).
Biochemical oxygen demand
10
One of the key water-quality parameters is the five-
day biochemical oxygen demand test (sometimes
referred to as the
biological oxygen demand
test,
or BOD
5
). This is a measure of the oxygen required
by bacteria and other micro-organisms to break
down organic matter in a water sample. It is an
indirect measure of the amount of organic matter in
a water sample, and gives an indication of how much
dissolved oxygen could be removed from water as
the organic matter decays.
The test is simple to perform and easily replic-
able. A sample of water needs to be taken, placed in
a clean, darkened glass bottle and left to reach 20°C.
Once this has occurred the dissolved oxygen content
should be measured (as a concentration). The sample
8
6
4
2
0
0
5
10
15
Time (days)
Figure 7.4
Dissolved oxygen curve. The solid line
indicates the dissolved oxygen content decreasing due
to organic matter. The broken line shows the effect of
nitrifying bacteria.