Geoscience Reference
In-Depth Information
interrelationship that exists between water quality
and water quantity in a river system.
One remarkable feature about rivers is that given
enough time and a reasonable pollution loading,
rivers will recover from the input of many pollutant
types. That is not to say that considerable harm can-
not be done through water pollution incidents, but
by and large the river system will recover so long as
the pollution loading is temporary. An example of
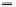
this can be seen in the
oxygen sag curve
(see Figure
7.2) that is commonly seen below point sources of
organic pollution (e.g. sewage effluent). The curve
shows that upon entering the river there is an
instant drop in dissolved oxygen content. This is
caused by bacteria and other micro-organisms in the
river feeding on the organic matter in the stream
and using any available dissolved oxygen. This
would have a severe impact on any aquatic fauna
unable to move away from this zone of low dissolved
oxygen. As the pollutant load moves downstream
the degradation, dilution and dispersal starts to take
effect and oxygen levels start to recover in the river.
The shape of the curve, especially the distance
downstream until recovery, is highly dependent on
the flow regime of the receiving river. A fast flowing,
readily oxygenated stream will recover much faster
than a slow-moving river. Large rivers will have a
faster recovery time (and the depth of sag will be
less) than small streams, due to the amount of
dilution occurring.
WATER-QUALITY PARAMETERS
To analyse the water quality within a river, con-
sideration has to be given to what type of test may
be carried out and the sampling pattern to be
used. There are numerous parameters that can be
measured, and each is important for the part they
play in an overall water-quality story. It is not
necessary to measure them all for a single water-
quality analysis study; instead the relevant
parameters for a particular study should be iden-
tified. This can be done using a priori knowledge
of the water-quality issues being studied. To aid in
this, different parameters are discussed here with
respect to their source; what type of levels might be
expected in natural rivers; and the impact they have
on a river ecosystem.
The first distinction that can be made is between
physical and chemical parameters. With chemical
parameters it is the concentration of a particular
chemical substance that is being assessed. With
physical parameters it is a physical measurement
being made, normally measuring the amount of
something within a water sample.
Physical parameters
Temperature
The temperature of water in a river is an important
consideration for several reasons. The most impor-
tant feature of temperature is the interdependence
it has with dissolved oxygen content (see p. 134).
Warm water holds less dissolved oxygen than colder
water. The dissolved oxygen content is critical in
allowing aquatic fauna to breathe, so temperature
is also indirectly important in this manner. Water
temperature is also a controlling factor in the rate
of chemical reactions occurring within a river.
Warm water will increase the rate of many chemi-
cal reactions occurring in a river, and it is able to
dissolve more substances. This is due to a weaken-
ing of the hydrogen bonds and a greater ability
of the bipolar molecules to surround anions and
cations.
100
80
60
40
20
0
0
200
400
600
800
Distance downstream
Figure 7.2
Hypothetical dissolved oxygen sag curve.
The point at which the curve first sags is the point
source of an organic pollutant. The distance down-
stream has no units attached as it will depend on the
size of the river.