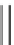Geoscience Reference
In-Depth Information
Fig. 4.3 General structure of
organophosphate pesticides
S (or O)
RO
P
Leaving Group
O
RO
Fig. 4.4 General structure of
N-methyl carbamate
O
H
3
C
Leaving Group
N
C
H
4.1.4 Triazines
Triazine pesticides and their metabolites are a group of closely related herbicides
used widely on agricultural and nonagricultural sites; they are inhibitors of elec-
tron transport in photosynthesis. As a family, their chemical structures are het-
erocyclic, composed of carbon and nitrogen in their rings. Most, except for
metribuzin, are symmetrical with their altering carbon and nitrogen atoms. Her-
bicide members in this family include atrazine, hexazinone, metribuzin, prometon,
prometryn, simazine, and their degradates. Atrazine is used widely in corn pro-
duction and is estimated to have been the most often-used pesticide in the USA
during the late 1990s. Its toxic effects may include disruption of ovarian function,
generation of mammary (breast) tumors in animals, and interference with the
binding of steroid hormones and the breakdown pathway of estrogen (Bradlow
et al.
1995
; Cooper et al.
1996
; Danzo
1997
). Some uses of atrazine are classified
as restricted because of groundwater and surface water concerns. Many of the
triazines show acute and chronic toxicities at low concentrations (Letterman
1999
;
Montgomery
1993
), and they generally are known or suspected to be carcinogenic,
mutagenic, and/or teratogenic (Newman
1995
; Letterman
1999
; Montgomery
1993
; C&EN
2000
,
2002
). Recent evidence (Reeder et al.
1998
; Renner
2002
;
Tavera-Mendoza et al.
2002a
,
b
) implicated specific triazines and/or their degra-
dation products as endocrine disruptors and teratogens in amphibians.
4.1.5 Paraquat and Diquat
Paraquat (1,1
0
-dimethyl, 4,4
0
-bipyridyl) is a nonselective contact herbicide. It is
used almost exclusively as a dichloride salt and usually is formulated to contain
surfactants. Both its herbicidal and toxicological properties are dependent on the
ability of the parent cation to undergo a single-electron addition, to form a free
radical that reacts with molecular oxygen to reform the cation and concomitantly