Geoscience Reference
In-Depth Information
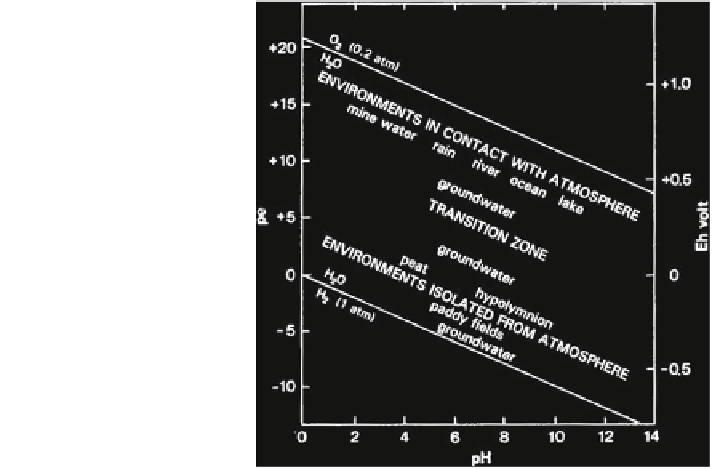
Fig. 2.4 The stability of
water and the ranges of pE
and pH conditions in natural
environments (Appelo and
Postma
1993
)
In many cases, redox reactions that are favorable from a thermodynamic point
of view may not actually take place: Sometimes, the activation energy barriers for
such reactions are too high to allow fast transformation, according to the preferred
thermodynamic considerations. For example, the complete oxidation of any
organic molecule to carbon dioxide and water is thermodynamically favorable.
However, such oxidation is not favorable kinetically, which implies that organic
molecules—including all forms of living species—are not oxidized immediately;
this fact explains the ability to sustain life. The reason for this difference between
kinetic and thermodynamic considerations, for redox reactions, is partly because
redox reactions are relatively slow compared to other reactions and partly due to
the fact that in many cases, reactions are poorly coupled because of slow species
diffusion from one microenvironment to another. Therefore, many redox reactions
are dependent on catalytic processes.
2.3 Adsorption
Adsorption is the net accumulation of matter on the solid phase at the interface with
an aqueous solution or gaseous phase. In this process, the solid surface is the
adsorbent and the matter that accumulates is the adsorbate. Adsorption also may be
defined as the excess concentration of a chemical at the subsurface solid interface
compared to that in the bulk solution, or the gaseous phase, regardless of the nature
of the interface region or the interaction between the adsorbate and the solid surface
that causes the excess. Surface adsorption is due to interactions between electrical
charges, or nonionized functional groups, on mineral and organic constituents.