Geoscience Reference
In-Depth Information
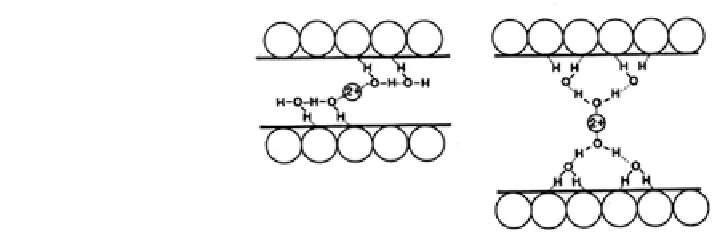
Fig. 18.21 Arrangement of
H
2
O chains linking a divalent
exchange cation to the
surfaces of a 2.0 nm hydrate
of smectite (modified after
Farmer
1978
, in McBride
1989
). Copyright 1989 Soil
Science Society of America.
Reprinted with permission
Interlayer expansion of layered clay is shown in Fig.
18.21
, where the
arrangements of H
2
O chains linking a divalent cation to the surface of a 2.0 nm
hydrate of smectite clay are characterized by 2:1 layers. The force-inducing clay
expansion arises from ion-dipole interactions, while the force-opposing clay
swelling emanates from electrostatic interactions. In general, the swelling of 2:1
silicates is considered to be induced by hydration of charge-compensating counter-
ions in the interlayer space of clay.
Laird (
2006
) defined six separate processes driving the swelling of smectite
clays saturated with alkali and alkaline earth cations in an aqueous system, as
follows: crystalline swelling, double-layer swelling, quasi-crystal formation, cat-
ion demixing, covolume swelling, and Brownian swelling. In an aqueous system,
some of these processes may act concurrently, affecting swelling dimension. An
increase in layer charge leads to a decrease in crystalline swelling, but increases
the size and stability of smectite clay quasicrystals. When two smectite quasi-
crystals approach each other in an aqueous suspension, a portion of their double
layer may fuse expelling anions, cations, and water from the contact surfaces. In
dilute aqueous suspensions, Na-smectite can be almost completely delaminated, a
diffuse double layer is formed between the individual layers, and each layer
behaves as a stable individual colloid (Sposito
1992
; Laird
2006
). A schematic
diagram
depicting
the
breakup
and
formation
of
quasicrystals
is
given
in
Fig.
18.22
, leading to an increase in soil-clay swelling.
In situ laser microscopy combined with digital image analysis was used by
Suzuki et al. (
2005
) to investigate the swelling of bentonite aggregates in a NaCl
solution of variable concentration. Changes in quasicrystal shape after contact with
NaCl solutions are shown in Fig.
18.23
. These results refer only to a simple NaCl
water solution. However, in natural soil-subsurface conditions, saline irrigation
water and groundwater contain also divalent cations, which can exchange with
Na
+
ions in bentonite. As a consequence, the results of Suzuki et al. (
2005
) are
significant only for understanding the mechanism of Na
+
-controlled swelling of
expandable soil clays.
Hysteresis was noted in investigations of clay swelling, beginning with the
early pioneering studies of Mooney et al. (
1952
) and Norrish (
1954
). Swelling
hysteresis was attributed to changes in the energy levels of expansion and