Geoscience Reference
In-Depth Information
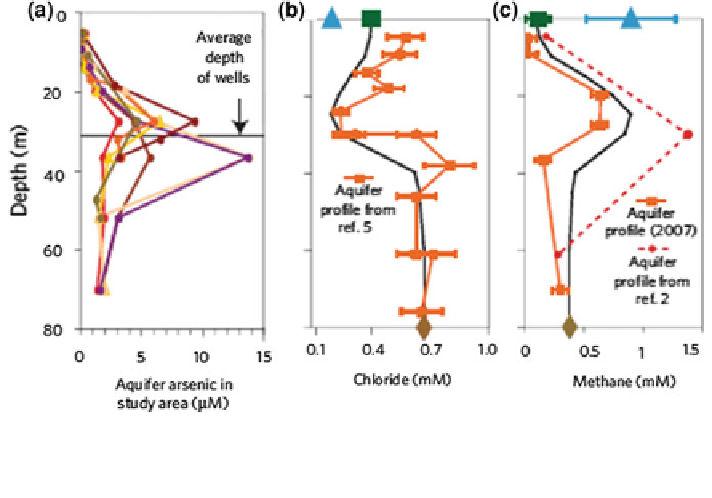
Fig. 17.16 Concentration and recharge profiles of a arsenic, b chlorides, and c methane in
groundwater from Munshiganj, Bangladesh in seven well clusters within a 0.03 km
2
(Neumann
et al.
2010
)
Contamination of groundwater by arsenic is discussed by Appleyard et al.
(
2006
), who examined As enrichment in groundwater in an urban area in Perth
(Australia) as a result of exposure of pyritic sediments caused by reduced rainfall
and increased groundwater exploitation. SEM analysis of framboidal pyrite within
the peat and sand sediments indicates the mineral to be a source of As in shallow
groundwater. The oxidation of arsenian pyrite, as described by Smith et al. (
2006
),
is:
Þ
ð
s
Þ
þ
7
=
2O
2
þ
H
2
O
$
Fe
3
þ
þ
SO
2
þ
AsO
3
4
þ
2H
þ
:
ð
FeS
As
4
Products of this reaction are Fe
3+
, arsenate as As(V) species, SO
4
2-
and H
+
ions. While no As was detected in groundwater in 1976, excessive dewatering and
peat excavation by 2004 led to peak arsenic concentrations in the groundwater,
ranging from 1,000 lg/L to 7,000 lg/L. Arsenic concentrations in shallow
groundwater fluctuated with time and location (Fig.
17.17
), but traces of As
always remained. The authors suggest that falling Eh values triggered the release
of As from the reduction of Fe(III) oxyhydroxide minerals near the base of the
unconfined aquifer, inducing continuous and essentially irreversible (at least on a
human lifetime scale) contamination of groundwater.
Groundwater contamination by inorganic speciation forms of arsenic and
selenium, following their leaching from a coal ash dumping ground (Poland), is
reported by Siepak et al. (
2004
). Groundwater concentrations of arsenic and
selenium, measured over 4 years, and at eight different sampling sites, are shown
in Table
17.3
. Maximum concentrations of arsenic and selenium were 128.8 and