Geoscience Reference
In-Depth Information
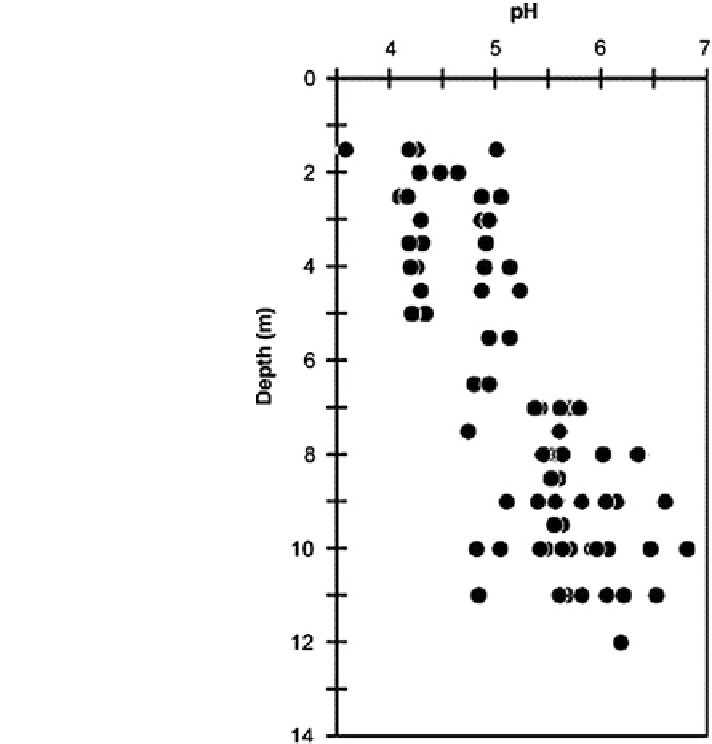
Fig. 17.9 Groundwater pH
downstream from a forest
area as a function of depth.
The data were compiled from
different multisampler wells
(Franken et al.
2009
).
Reprinted from Franken et al.
(
2009
). Copyright (2009)
with permission from
Elsevier
Franken et al. (
2009
) suggest that the low pH may be associated with the
mobilization of Al
3+
by dissolution of gibbsite and amorphous Al(OH)
3
:
Al O
ð
3
þ
3H
þ
$
Al
3
þ
þ
3H
2
O
or, alternatively, that the pH is in equilibrium with the mineral jurbanite according
to the reaction
Al O
ð
SO
4
$
Al
3
þ
þ
OH
þ
SO
2
4
:
Calculating the saturation index (SI) of groundwater for the above minerals, it is
seen (Fig.
17.10
) that for jurbanite, the SI of the groundwater remains close to zero
over the entire pH range. This indicates that equilibrium with jurbanite controls the
pH and Al
3+
concentration in the groundwater and that an accumulation of SO
4
2-