Geoscience Reference
In-Depth Information
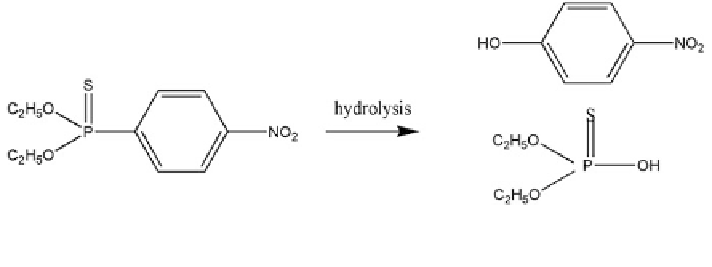
Fig. 16.35 Pathway of parathion degradation in soil. Reprinted from Nelson et al. (
1982
).
Copyright 1982 with permission of Elsevier
16.2.2 Pesticides
Crop protection chemicals are an important group of contaminants that exhibit
biologically mediated transformation in aerobic or anaerobic subsurface environ-
ments. We consider two well-known contaminants: the insecticide parathion,
which is an organophosphate compound, and the herbicide atrazine, from the
triazine group.
Parathion (O,O-diethyl O-p-nitrophenyl phosphorothioate) is degraded in the
near-subsurface aerobic environment via hydrolysis, where two degradation
products are observed, diethylthiophosphoric acid and p-nitrophenol, according to
the schematic pathway described in Fig.
16.35
. Abiotic hydrolysis of parathion in
the subsurface is a result of a surface-mediated transformation (see Sect.
16.1
)ora
biodegradation process.
Hydrolysis of parathion in a loessial semiarid soil was investigated by Nelson
et al. (
1982
). They found that Arthrobacter sp. hydrolyzed parathion rapidly in
sterilized, parathion-treated soil under aerobic conditions (20 % w/w water con-
tent). This bacterium was isolated from a silty loam, sierozem soil of loessial
semiarid origin (Gilat). It uses parathion or its hydrolysis product, p-nitrophenol,
as the sole carbon source. However, when parathion hydrolysis causes the amount
of p-nitrophenol to reach a concentration greater than 1 mM or if the concentration
is greater than 1 mM in the case of a single application of p-nitrophenol, the
hydrolysis product becomes noxious to the bacteria and their growth is inhibited.
In an accompanying laboratory study on the kinetics of biologically induced
hydrolysis of parathion, Nelson et al. (
1982
) found that bacteria populations
increased to a maximum four to five days after parathion application, with the
increase proportional to the concentration of parathion, followed by a decline.
Figure
16.36
shows this behavior in remoistened Gilat soil after application of
parathion in amounts of 10-160 lg/g dry soil.
The rate of parathion hydrolysis is independent of the parathion concentration;
the rate of formation of the hydrolysis product, P, is described by
dP
=
dt ¼ 1
=
Y dm
=
dt
þ
A
ð
m
m
o
Þ
ð
16
:
5
Þ