Geoscience Reference
In-Depth Information
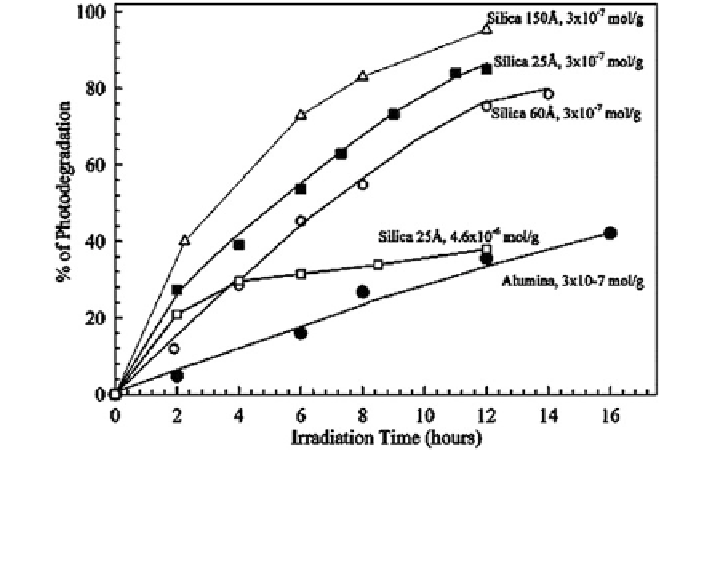
Fig. 16.18 Effect of surface nature and loading on the photodegradation rate of adsorbed BeP.
The photolysis experiments were carried out in a rotary cell, and the BeP remaining in the sample
was determined by HPLC. Uncertainties in measurements are in the range of 4-10 % of the
absolute values. Reprinted with permission from Fioressi and Arce (
2005
). Copyright 2005
American Chemical Society
have been detected in the environment. After exposure to the land surface, these
contaminants adsorb on soil materials and may reach the atmosphere as particulate
matter; these particulates are subsequently subject to photolytic reactions. In this
context, Ahn et al. (
2006
) studied photolysis of BDE-209 adsorbed on clay min-
erals, metal oxides, and sediments, under sunlight and UV dark irradiation. Dark
and light control treatments during UV and sunlight irradiation showed no disap-
pearance of BDE-209 during the experiments. Data on half-lives and rate constants
of BDE-209 adsorbed on subsurface minerals and sediments, as determined by Ahn
et al. (
2006
) and extracted from the literature, are shown in Table
16.6
.
As a general pattern, we see that photodegradation is greater under UV light
than sunlight irradiation. After 100 days of sunlight irradiation, the half-lives of
BDE-209 are 216 days adsorbed on montmorillonite, 408 days adsorbed on kao-
linite, and 990 days adsorbed on sediments. There are no significant losses of
BDE-209 adsorbed on aluminum hydroxide, ferrihydrite, or birnessite. The longer
half-life of BDE-209 adsorbed on sediments is explained by the presence of
organic matter. Organic matter may inhibit photodegradation of organic chemicals
either by shielding the contaminant from available light or by quenching the
excited states
of the organic molecules before they react to form products
(Bachman
and
Patterson
1999
).
Fourteen
days
of
UV
irradiation
cause
the