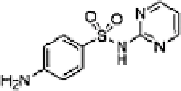Geoscience Reference
In-Depth Information
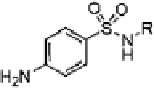
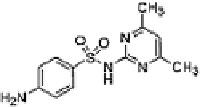
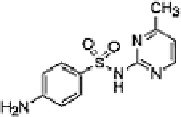
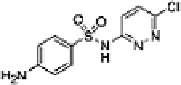
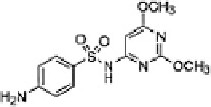
Table 16.5 General structure of a sulfa drug, structure of five sulfa drugs containing six-
membered heterocyclic R substituents, and measured pK
a
values; pK
a
values were calculated
using a spectrophotometry titration
Compound
General structure
a
a
Structure
pk
a,1
pk
a,2
1 Sulfamethazine
2.6 ± 0.2
8 ± 1
2 Sulfamerazine
2.5 ± 0.7
7 ± 1
3 Sulfadiazine
2 ± 1
6.4 ± 0.6
4 Sulfachloropyridazine
2 ± 3
5.9 ± 0.3
5 Sulfadimethoxine
2.9 ± 0.5
6.1 ± 0.2
Errors represent the 95 % confidence levels, and both the pK
a
values and the associated errors
were obtained from fits of the data using Scientist for Windows. Reprinted with permission from
Boreen et al. (
2005
). Copyright 2005 American Chemical Society
both direct and indirect photodegradation, for all five studied compounds, was
identified as a sulfur dioxide extrusion product. Based on accurate mass balance,
Boreen et al. (
2005
) suggest several structures for the photoproducts, such as 4-(2-
imino-4,6-dimethilpyridimin-1-(2H)-yl)aniline derived from sulfamethazine.
Photochemical transformation of benzole (e)pyrene (BeP), a polycyclic aro-
matic hydrocarbon, adsorbed on silica gel and alumina surfaces, is reported by
Fioressi and Arce (
2005
). This study was designed to define the atmospheric
degradation of the PAH adsorbed on particulates that originated from wind erosion
of the land surface. It can be assumed that similar behavior occurs at the land
surface-atmosphere interface.