Geoscience Reference
In-Depth Information
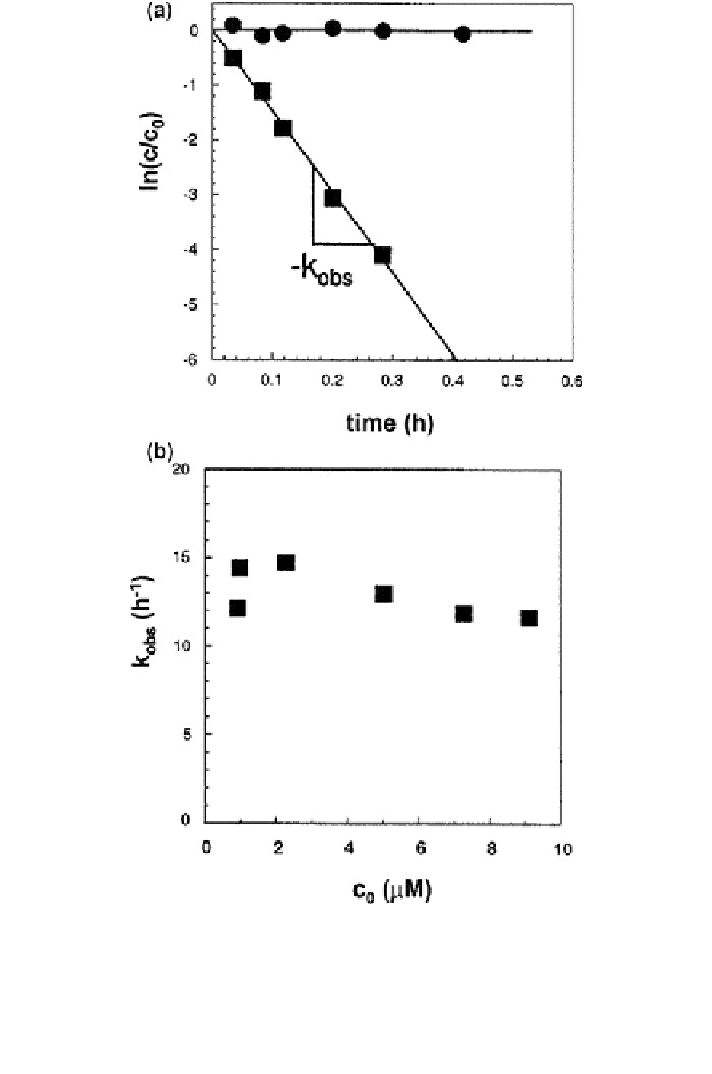
Fig. 16.11 Evaluation of the rate law of the reduction of CBr
2
Cl
2
by Fe(II) in suspensions of
goethite (25 m
2
/L; Fe(II)
tot
of 1 mM; pH 7.2; ionic strength 20 mM; at 25 C). The contact time
of Fe(II) with iron oxide before addition of PHM was [24 h. a semilogarithmic plot of relative
concentration (CBr
2
Cl
2
, c
0
= 2.3 lM) versus time in the presence (filled square box) and
absence (filled circle) of goethite; b plot of pseudo-first-order rate constants for CBr
2
Cl
2
, k
obs
,
versus initial concentrations of CBr
2
Cl
2
in suspension of Fe(II) and goethite. Reprinted with
permission from Pecher et al. (
2002
). Copyright 2002 American Chemical Society