Geoscience Reference
In-Depth Information
Table 16.1
Characteristics of the investigated soils and parameters affecting redox of chromium
Soil type
Oxidative
capacity of
soil for Cr(III)
(lg/g)
Clay 22.0 7.3 1.52 6.8E-5 \5.0 57.7 399 8.96
Peat 50.2 7.0 41.1 4.3E-4 \5.0 57.7 60 \0.05
Sand 13.0 7.4 3.0 1.7E-4 \2.5 48.8 150 3.48
Cambisols 24.4 5.4 0.22 4.6E-5 \5.0 144 234 7.44
TOC Total Organic Carbon, SOM Soluble Organic Matter. Reprinted with permission from Kozuh et al. (
2000
).
Copyright 2000 American Chemical Society
Moisture
(%)
pH of
soil
TOC
(%)
SOM
(%)
Exchangeable
Cr (lg/g)
Total Cr
(lg/g)
Exchangeable
manganese (IV)
oxides (lg/g)
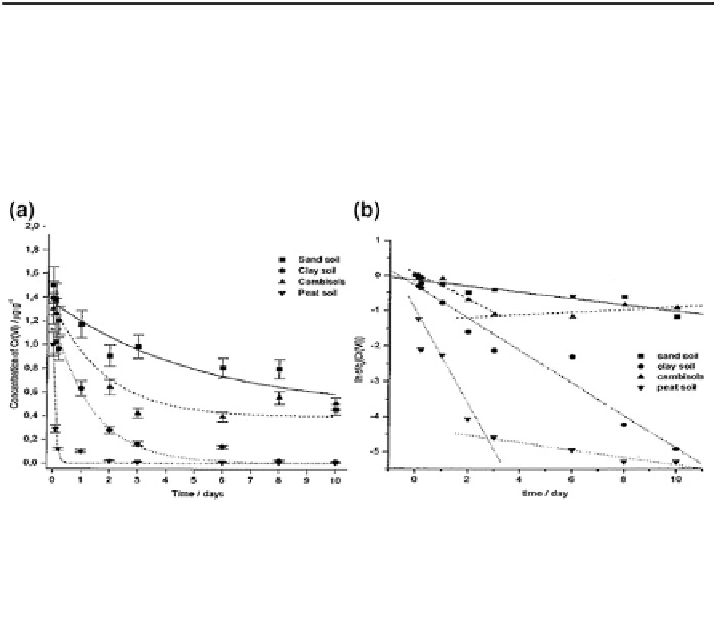
Fig. 16.3 a Kinetics of reduction of soluble Cr(VI) added concentrations of 1 lg/g Cr(VI) in
various soils and b first-order plots of ln[relative Cr(VI) concentration] versus time for the
reduction of Cr(VI) in various soils. The slope of the lines is equal to the first-order rate constants
(k
exp
) for the case of 1 lg/g Cr(VI) added. Reprinted with permission from Kozuh et al. (
2000
).
Copyright 2000 American Chemical Society
chromium in the soils. Therefore, the results of Kozuh et al. (
2000
) confirm that
reduction and oxidation of soluble chromium can occur in natural soils, but that
reduction of Cr(VI) dominates over oxidation of Cr(III). The composition (organic
matter and presence of Mn(IV) oxides) of the soils and environmental conditions
affect the extent of these processes.
Arsenic contaminants may be found in the aquatic and terrestrial environments
as a result of anthropogenic inputs and weathering of primary materials. It is
known (e.g., Oscarson et al.
1983
; Tournassat et al.
2002
) that in such environ-
ments, manganese oxides like birnessite (d-MnO
2
) directly and rapidly oxidize
As(III) to As(V). However, As(III) oxidation can be inhibited in sediments when
additional natural materials lead to coating of MnO
2
by CaCO
3
(Oscarson et al.
1983
).
Power et al. (
2005
) show the effect of pH and initial As(III) concentration on
the kinetics of arsenite oxidation at birnessite-water interfaces, when a competi-
tive metal (e.g., Zn) is present in an adsorbed or nonadsorbed state (Fig.
16.5
).