Geoscience Reference
In-Depth Information
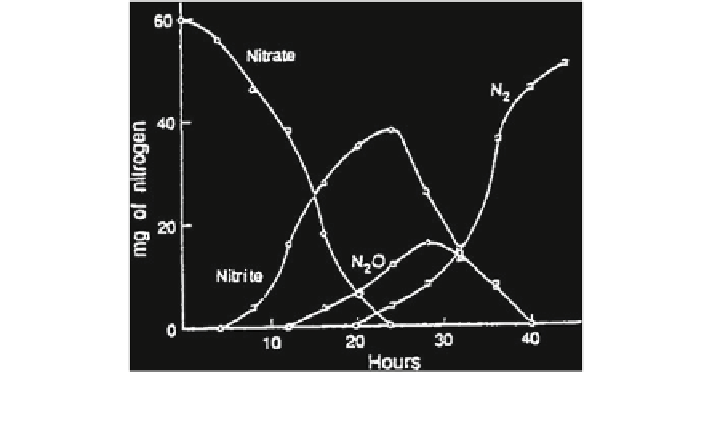
Fig. 15.8
Products formed during denitrification in Melville loam, pH 7.8 (Cooper and Smith
1963
)
the soil bacterium that oxidizes nitrite NO
2
-
to NO
3
-
. In most habitats, this
bacterium is found together with Nitrosomonas, Nitrospira, or Nitrosovibrio,
which oxidize ammonia (NH
3
) to the nitrite required for NO
3
-
formation. Nitri-
fication is affected by the subsurface pH (with an optimum value varying between
6.6 and 8.0) and the subsurface water-air ratio. Once NO
3
-
is formed, it becomes
subject to transformation by microorganism-mediated denitrification to gaseous
oxides of nitrogen and to N
2
.TheNO
3
-
may be taken up by organisms and used in
synthesis of amino acids (assimilatory reduction), or in the absence of O
2
, it may
be used by microorganisms as an electron acceptor by reduction to NH
4
+
(Paul and
Clark
1989
). Enzymatic denitrification is the result of assimilatory reduction of
NO
3
-
by microorganisms and dissimilatory reduction of nitrate to ammonium.
This is accomplished by specific organisms in the absence of O
2
. Figure
15.8
shows the following sequence of identifiable products formed during denitrifica-
tion: NO
3
-
;NO
2
-
;N
2
O; N
2
.
Under field conditions, not all intermediate products are converted to N
2
.
Nitrate reductase, for example, causes a decrease in the enzymatic activity.
Denitrificaton in the absence of oxygen is caused by a large number of bacteria;
Table
15.4
lists the main microorganisms capable of denitrification.
Denitrification occurs only in the presence of oxidized nitrogen and in an
environment with limited O
2
(which prevails in the subsurface). Because deni-
trification is an enzyme-mediated reaction, the substrate concentration functions as
a rate-determining factor. The dominant denitrifying bacteria are heterotrophic.
The favored environmental conditions for the growth of denitrifying bacteria
include a neutral pH (6-8), a favorable water-air (oxygen) ratio, and a subsurface
temperature between 20 and 30 C.
Phosphorus in the subsurface originates from a natural parent material or
anthropogenic application on land surface (e.g., fertilizers, pesticides, surfactant