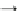Geoscience Reference
In-Depth Information
Table 15.2 Oxidation reactions in microbial pesticide metabolism (Bollag and Liu
1990
)
Hydroxylation
RCH ? RCOH
ArH ? ArOH
N-dealkylation
RNCH
2
CH
3
? RNH ? CH
3
CHO
ArNRR
0
? ArNH
2
b-Oxidation
ArO(CH
2
)
n
CH
2
CH
2
COOH ? ArO(CH
2
)
n
COOH
Decarboxylation
RCOOH ? RH ? CO
2
ArCOOH ? ArH ? CO
2
Ar
2
CH
2
COOH ? Ar
2
CH
2
? CO
2
Ether cleavage
ROCH
2
R
0
? ROH ? R
0
CHO
ArOCH
2
R ? ArOH ? R
0
CHO
Epoxidation
O
RCH = CHR' RCH-CHR'
Oxidative coupling
2ArOH ? (Ar)
2
(OH)
2
Sulfoxidation
RSR
0
? RS(O)R
0
or RS(O
2
)R
0
phases. Reductive reactions occurring in a saturated subsurface, due to microbial
activity, can lower the redox potential to a range of 0 to -100 mV (Parr and Smith
1976
) and lead to the transformation of organic chemicals.
Oxidation of organic contaminants by microorganisms is one of the basic
metabolic reactions in the subsurface and involves the presence of a group of
oxidative enzymes such as peroxidases, lactases, and mixed-function oxidases.
Major oxidative reactions that may occur in the subsurface are presented and
explained in Table
15.2
.
Hydroxylation can occur on the aromatic ring, on aliphatic groups, and on alkyl
side chains. It makes these compounds more polar, so that their solubility
increases. Hydroxylation (Fig.
15.1
) is one of the most common first steps in
contaminant transformation, and it begins by the addition of an hydroxyl group.
N-dealkylation results from an alkyl substitution on an aromatic molecule,
which is one of the first places where microorganisms initiate catabolic transfor-
mation of atrazine, a xenobiotic molecule (Fig.
15.2
). It is a typical example of a
reaction leading to transformation of pesticides such as phenyl ureas, acylanilides,
carbamates, s-triazines, and dinitranilines. The enzyme mediating the reaction is a
mixed-function oxidase, requiring a reduced nicotinamide nucleotide as an H
donor.