Geoscience Reference
In-Depth Information
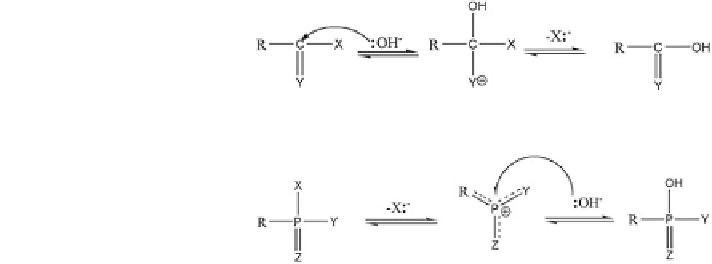
Fig. 14.2 Illustration of the
S
N
2 mechanism (nucleophilic
substitution) for hydrolysis
reaction (Huang
2000
)
Fig. 14.3 Illustration of the
S
N
1 mechanism (nucleophilic
substitution) for hydrolysis
reaction (Huang
2000
)
amides, anilides, and phosphate-containing esters. Metal ions and protons coor-
dinate to the organic contaminant, so that electron density is shifted away from the
site of nucleophilic attack to facilitate the reaction.
Metal ion-induced catalysis generally occurs via complexation of the reactant
molecule. Stone et al. (
1993
) formulate a general pathway of the process as fol-
lows: (1) Complex formation constants increase as the charge-to-radius ratio of the
metal ion increases, (2) polarizable metals and ligands exhibit complex stability
through covalent bond formation, and (3) competition for the metal among
available ligands becomes greater as complex formation constants increase. The
properties of metal ions, in general, and transition metal ions, in particular, make
them good catalysts for a broad range of organic and inorganic reactions in the
subsurface environment. These reactions are described at length in the literature
(e.g., McBride
1994
; Smolen and Stone
1998
). Catalysis by surface-bound metals
is observed when all participating reactants are significantly adsorbed, and when
rate constants for the reaction at the solid-liquid interface exceed those in the
surrounding liquid phase. Lewis acid properties of metals, for example, are sig-
nificant in mineral-catalyzed hydrolysis reactions. Mineral-catalyzed degradation
occurs mainly for the hydrolysable organics that have a structure suitable for
complexation with the surface metal cations.
In an extensive review on abiotic catalysis, Huang (
2000
) noted that the
reactivity of hydrolyzable organic contaminants arises from the presence of
electron-deficient (electrophilic) sites within the molecules. Figures
14.2
and
14.3
show the patterns of reactivity in two cases of nucleophilic substitution and
monomolecular nucleophilic substitution. The S
N
2 mechanism (nucleophilic
substitution) involves attack of the electrophilic sites by OH
-
or H
2
O, generation
of a higher coordination number intermediate, subsequent elimination of the
leaving group, and the formation of an hydrolysis product (Fig.
14.2
).
In the case of monomolecular nucleophilic substitution (S
N
1), the reaction
proceeds with the loss of the leaving group to generate a lower coordination
number
intermediate,
followed
by
generation
of
the
hydrolysis
product
by
nucleophilic addition, as shown in Fig.
14.3
.