Geoscience Reference
In-Depth Information
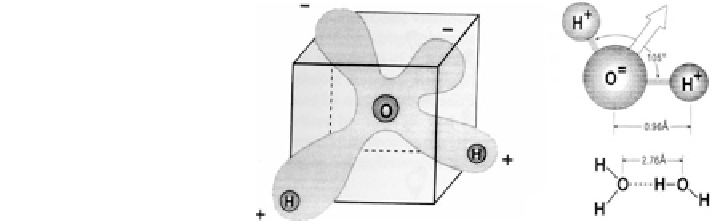
Fig. 1.10 Water as a solvent:
electron cloud depiction for a
water molecule and the
structure of the angular water
molecule and hydrogen bond
(Horne
1969
)
1.2 Subsurface Liquid Phase
Within the subsurface zone, two liquid phase regions can be defined. One region,
containing water near the solid surfaces, is considered the most important surface
reaction zone. This ''near solid phase water,'' which is affected by the solid phase
properties, controls the diffusion of the mobile fraction of the solute adsorbed on
the solid phase. The second region constitutes the ''free'' water zone, which
governs liquid and chemical flow in the porous medium.
The composition and reactivity of the liquid phase (known as the soil solution)
is defined by the quality of the incoming water and affected by fluxes of matter and
energy originating from the vicinity of the solid phase, microbiological activity,
and the gas phase. To understand the properties of the subsurface liquid phase, it is
first necessary to consider the structure of the water molecule.
The H
2
O molecule has a dipolar character with a high negative charge density
near the oxygen atom and a high positive charge density near the protons.
Figure
1.10
depicts the electron cloud of the angular water molecule resulting
from the hybridization of electrons, to yield two bonding orbitals between the
O and the two H atoms. This specific character strongly influences the interaction
of water with the solid and air phases in the subsurface.
The local structure of water has been compared to ordinary hexagonal ice
structures and calculated spectra. Synchrotron X-ray measurements have led to
contrasting opinions regarding the H-bond coordination environment in liquid
water. Wernet et al. (
2004
) used this technique, together with X-ray Raman
scattering, to probe the molecular arrangement in the first coordination shell of
liquid water. Most molecules in liquid water are in two hydrogen-bonded con-
figurations with one strong donor and one strong acceptor hydrogen bond, in
contrast to the four hydrogen-bonded tetrahedral structure specific to ice. Heating
water to 90 C causes about 10 % of the molecules to change from tetrahedral
environments to two hydrogen-bonded configuration.
Water is a dynamic liquid, where H-bonds are continuously broken and
reformed. Wernet et al. (
2004
) show that water, probed on the subfemtosecond
time scale, consists mainly of structures with two strong H-bonds, implying that
most molecules are arranged in strongly H-bonded chains, or in rings embedded in
a disordered cluster network connected mainly by weak H-bonds. These results are