Geoscience Reference
In-Depth Information
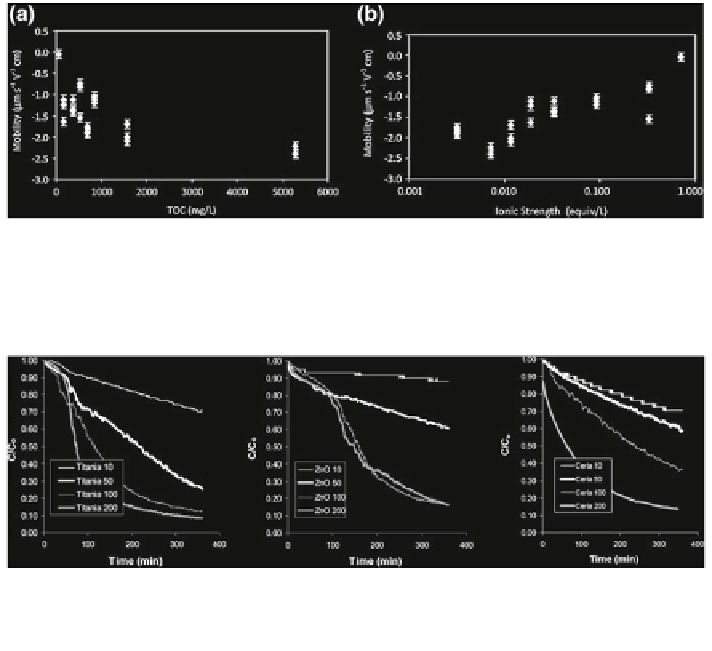
Fig. 12.15 Electrophoretic mobility of TiO
2
, ZnO, and CeO
2
in nine different waters as a
function of a TOC; and b ionic strength (Keller et al.
2010
). Reprinted with permission from
Keller et al. (
2010
). Copyright 2010 American Chemical Society
Fig. 12.16 Sedimentation of TiO
2
, ZnO, and CeO
2
in seawater at 4 different initial nanoparticle
concentrations (10, 50, 100, and 200 mg/L) (Keller et al.
2010
). Reprinted with permission from
Keller et al. (
2010
). Copyright 2010 American Chemical Society
The electrophoretic mobilities of these metal oxide nanoparticles, in aqueous
solutions of different origin and composition, are presented in Fig.
12.15
. The
electrophoretic mobility was dependent on the presence of natural organic matter
(represented by Total Organic Carbon, TOC) (Fig.
12.15
a) and ionic strength
(Fig.
12.15
b), and not affected by the pH (data not shown). This observation was
explained by the following trends: (1) as TOC concentration increases, the charge
on the nanoparticles becomes more negative, and (2) as ionic strength increases,
the nanoparticle charge is neutralized more effectively.
Keller et al. (
2010
) report that the aqueous composition affects the sedimen-
tation and aggregation status of TiO
2
, ZnO, and CeO
2
. The highest rate of sedi-
mentation was observed in an aqueous environment characterized by low TOC and
high ionic strength; note that high concentrations of suspended nanoparticles were
considered. The rate of sedimentation decreased rapidly as nanoparticle aggregates
were removed from solution, lowering the concentration of dispersed nanoparticles
(Fig.
12.16
). The chemistry of the aqueous solution controls the extent of sedi-
mentation. The availability of organic molecules which may be adsorbed on the