Geoscience Reference
In-Depth Information
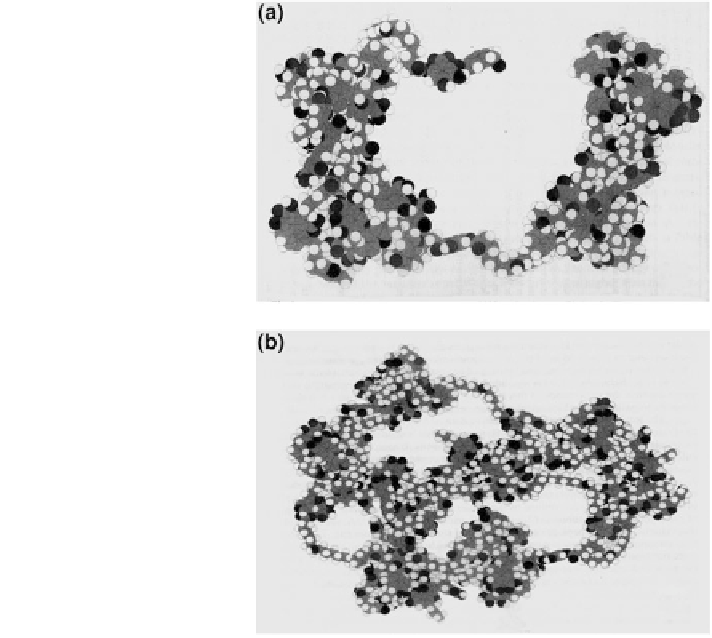
Fig. 1.9 Terrestrial humic
acid model a tetramer open
form, and b trimer trapping
an additional monomer
(Schulten
2001
)
angle, dihedral, van der Waals, stretch bend and electrostatic energy derivatives,
and tentatively association energy. Atomic and molecular properties were calcu-
lated at the nanometer level. Schulten (
2001
) compared the covalently bound
tetramer humic acid, which is geometrically optimized in a widely open molecular
structure (Fig.
1.9
a), to the tetramer design formed by trapping a monomer in a
trimer humic acid structure (Fig.
1.9
b). Both molecules have the same number of
atoms, molecular weight, and elemental composition. Surface areas, volumes, and
density are quite similar in the wide-open and trapped tetramer forms. The only
substantial difference is that the accessible surface area is much larger in the open
(tetramer) system. Natural organic matter is considered by Schaumann (
2006
)asa
three-dimensional polymer-like, entangled phase of macro- and micromolecules
having a pH-dependent charge. The molecular size range is defined by the natural
organic matter form (dissolved or solid) and age (young or old) and is affected by
various environmental factors.
These types of models, while incomplete, are steps toward the formulation of
composite models, which depend on future availability of compositional data.
Moreover, these structural models are an important aid in understanding the
interactions between anthropogenic chemicals and terrestrial organic matter.
However, due to the heterogeneity of humic substances in the environment,