Geoscience Reference
In-Depth Information
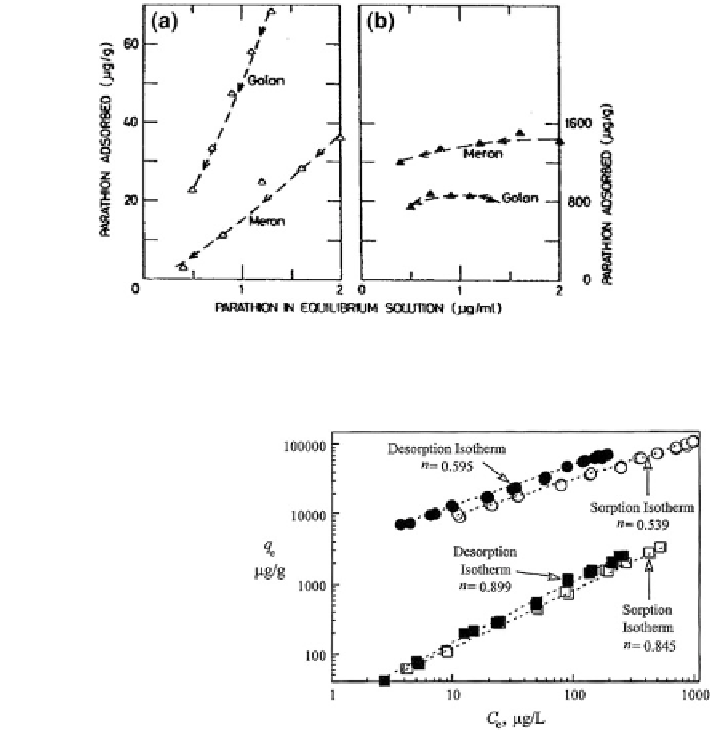
Fig. 8.49 Calculated parathion desorption from the a mineral and b organic fractions of Golan
and Meron soils. Saltzman et al. (
1972
). Copyright 1972 American Chemical Society
Fig. 8.50 Phenanthrene
adsorption-desorption
hysteresis observed for
Lachine and Chelsa humic
acid aggregate; the hysteresis
index is given by n. Reprinted
from Huang et al. (
2003
).
Copyright 2003 with
permission of Elsevier
solution) of acetonitrile solvent from homoionic montmorillonite clays is reversible,
and hysteresis appears to exist except for K
+
-montmorillonite. This behavior sug-
gests that desorption may be affected by the fundamental difference in the swelling
of the various homoionic montmorillonites, when acetonitrile is present in the water
solution. During adsorption, it was observed that the presence of acetonitrile affects
the swelling of different homoionic clays. At a concentration of 0.5 M acetonitrile in
solution, the layers of K
+
-montmorillonite do not expand as they would in pure
water, while the layers of Ca
2+
- and Mg
2+
-montmorillonite expand beyond a par-
tially collapsed state. The behaviors of K
+
-, Ca
2+
-, and Mg
2+
-montmorillonite are
different from the behavior of these clays in pure water. Na
+
-montmorillonite is not
affected by acetonitrile presence in an aqueous solution.
The heterogeneity of subsurface OM also may influence the release of adsorbed
organic contaminants. Huang and Weber (
1998
) present adsorption-desorption
isotherms of phenanthrene for a kerogen (Lachine) and a humic acid (Chelsea).