Geoscience Reference
In-Depth Information
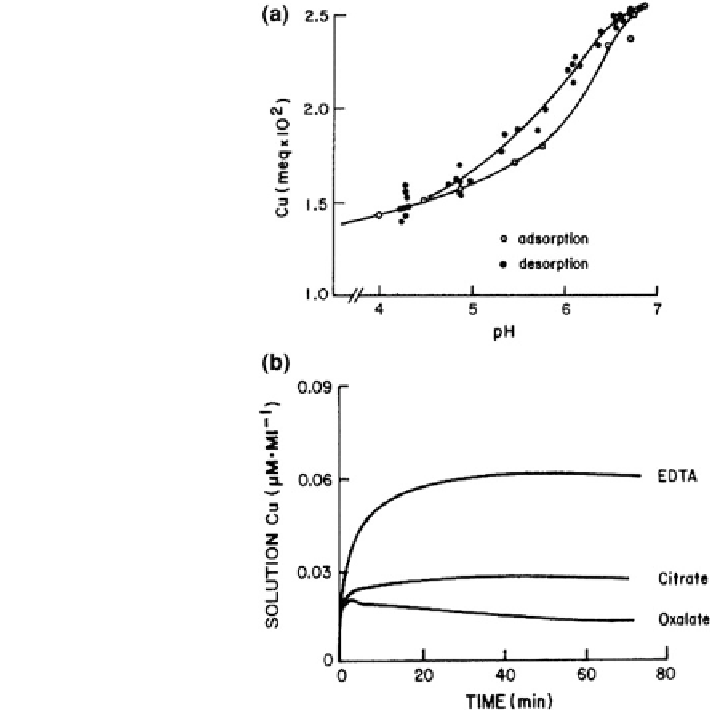
Fig. 8.47 Sorption of Cu
2+
on montmorillonite as a
function of a pH and b time-
dependent release of soil-
absorbed Cu by EDTA,
citrate, and oxalate (Calvet
1989
; Lehman and Harter
1984
)
A difference in the rate of adsorption and desorption of Cr(VI) by alluvium was
also observed in a batch experiment (Fig.
8.46
b). On the basis of these two
experiments, Stollenwerk and Grove (
1985
) concluded that the quantity of Cr(VI)
adsorbed by alluvium is a function of its concentration as well as of the type and
concentration of other anions in solution. The Cr(VI) adsorbed through nonspecific
processes is desorbed readily by a Cr-free solution. Stronger bonds that are formed
between Cr(VI) and alluvium during specific adsorption result in very slow release
of this fraction. The Cr(VI) desorption from the alluvium material illustrates the
hysteresis process that results from chemical transformation of a portion of con-
taminant retained in the subsurface.
The release rate of ''nondesorbable,'' metallic cation contaminants adsorbed on
the solid phase can be examined in terms of three environmental factors—pH, the
presence of another metallic cation, and ligand presence—all of which vary
substantially in subsurface aqueous solutions. A decrease in pH favors the release
of cationic metals adsorbed on surfaces. An example of pH effects on Cu
2+
release