Geoscience Reference
In-Depth Information
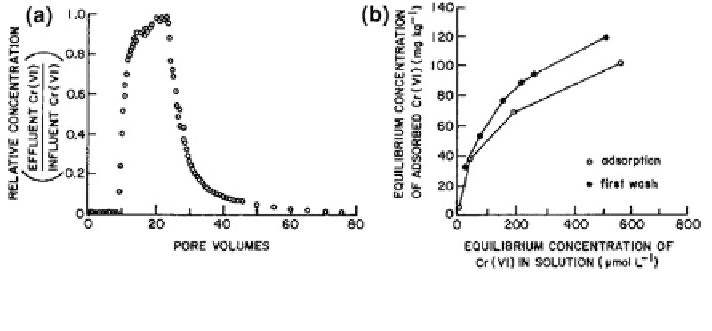
Fig. 8.46 Adsorption and desorption of Cr(VI) by a telluride alluvium in a a flow-through
column experiment and b on alluvium under batch conditions (Stollenwerk and Grove
1985
)
influenced by surface configuration, intraparticle diffusion, and the collapse of
edge-interlayer sites in solutions of K
+
,Rb
+
, and NH
4
. Moreover, Liu et al. (
2003
)
showed that only 40 % of the
137
Cs
+
adsorbed in a subsurface sediment, con-
taminated over a 30-year period, was desorbed by exchange with the electrolyte
solutions. This value increased up to 80 % after long-term contact with acidified
ammonium oxalate. Desorption studies with Cs
+
-spiked pristine sediment, equil-
ibrated over short duration, indicated that adsorbed Cs
+
is fully exchangeable with
Na
+
solution but becomes less exchangeable when placed in K
+
and Rb
+
elec-
trolyte solutions. This effect was attributed to the collapses of edges and partially
expanded interlamellar regions, which result from saturation of the exchange
complex with poorly hydrated Rb
+
and K
+
cations.
In many cases, a trace element retained on the subsurface solid phase may
undergo chemical reactions that induce a hysteresis phenomenon during the
release process. A relevant example of hysteresis due to precipitation of some of
the initial contaminants is given by the behavior of Cr(VI), an industrial con-
taminant, which in the subsurface environment may be subject to reduction
reactions. When an available source of electrons is present, such as OM, Cr(VI) is
reduced to Cr(III); the rate of this reaction increases with decreases in pH (Ross
et al.
1981
).
Stollenwerk and Grove (
1985
) report the adsorption and desorption of Cr(VI) in
an alluvial aquifer. From Fig.
8.46
a, we see that, over the first *10 pore volumes,
all the Cr(VI) in water contaminant was adsorbed by the alluvium. A rapid
increase in the effluent concentration of Cr(VI) then occurred, until the capacity of
alluvium for contaminant retention was exhausted (*25 pore volumes). Leaching
the alluvium column with 10 pore volumes of Cr-free water caused the release of
about 50 % of the adsorbed Cr(VI), and further leaching with 80 pore volumes of
groundwater, over 232 days, removed only an additional 34 % of the adsorbed
contaminant. Stollenwerk and Grove (
1985
) attributed the difficulty in removing
part of the adsorbed Cr(VI) to the presence of specific adsorption sites and possible
reduction to Cr(III) followed by precipitation.