Geoscience Reference
In-Depth Information
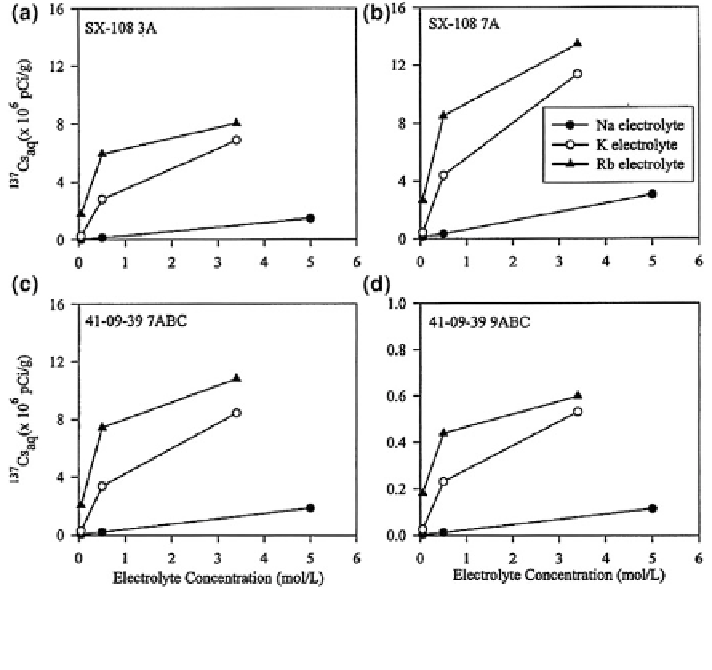
Fig. 8.45
137
Cs
+
desorption from the contaminated Hanford sediments after 6 days of
equilibration, as a function of exchanging cations and their concentrations. Reprinted from Liu
et al. (
2003
). Copyright 2003 with permission of Elsevier
in the concentration of metallic cations in solution, even if there is no modification
in the background electrolyte concentration. Release may also result from the
addition of protons or complexing molecules to the background electrolyte. This
process sometimes is used as an extraction technique for metallic cations, which
render mobile some strongly sorbed cations; but their passage into the solution
implies partial destruction of the solid phase.
Liu et al. (
2003
) studied radiocesium desorption from subsurface pristine and
contaminated micaceous sediments at the Hanford site, United States. Some of
these sediments were, in the past, contaminated accidentally by nuclear wastes
containing alkaline
137
Cs
+
. The desorption of
137
Cs
+
was measured in solutions of
Na
+
,K
+
,Rb
+
, and NH
4
electrolytes of variable concentration and pH and in the
presence of a strong Cs
+
-specific sorbent. Desorption of
137
Cs
+
from the con-
taminated sediment exhibits two distinct phases: an initial instantaneous release
followed by a slow kinetic process (Fig.
8.45
). The extent of
137
Cs
+
desorption
increases
with
increasing
electrolyte
concentration,
following
the
trend
Rb
+
[ K
+
[ Na
+
at a neutral pH. The extent and rate of
137
Cs
+
desorption is