Geoscience Reference
In-Depth Information
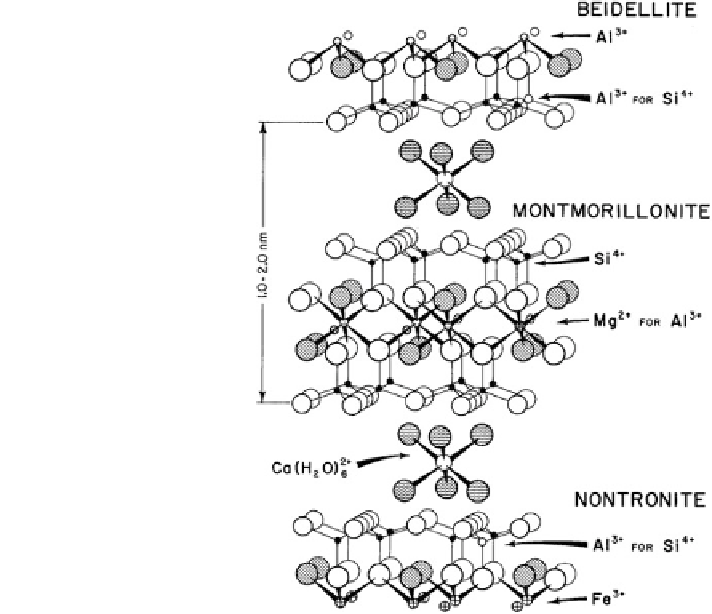
Fig. 1.5 The crystal
structure of smectite,
illustrating beidelite,
montmorillonite, and
nontronite (Borchard
1989
,
Brindley and MacEwan
1953
)
Of the naturally existing smectites, montmorillonite is a clay of major interest
in the subsurface environment. Figure
1.5
shows the crystal structure of mont-
morillonite, compared to those of beidellite and nontronite, and their possible
substitutions. Montmorillonite has an octahedral sheet that shares oxygen atoms
between two tetrahedral sheets. Cationic substitution may occur in the octahedral
or tetrahedral sheets.
Smectites are classified according to differences in properties and chemical
composition. For example, a typical formula for montmorillonite is Si
4
Al
1.5
Mg
0.5
with a cation exchange capacity of 135.5 cmol
c
/kg, while a typical formula for
beidellite is Si
3.5
Al
0.5
Al
2
with a cation exchange capacity of 135.2 cmol
c
/kg. Note
that montmorillonites contain significant amounts of tetrahedral Al and octahedral
Fe. Layer separation in smectite depends both on the interlayer cation and the
amount of water associated with the cation. The interlayer cations are replaced
when the clay is wetted with an electrolyte solution, and this affects the interlayer
spacing. The hydration water of the exchangeable cation forms the first layer, and
an additional water layer is held with less energy (Barshad
1960
). Changes in the
hydration status of smectites, as a result of an increase in ambient temperature, are
determined by differential thermal analysis (DTA). Smectites lose water that