Geoscience Reference
In-Depth Information
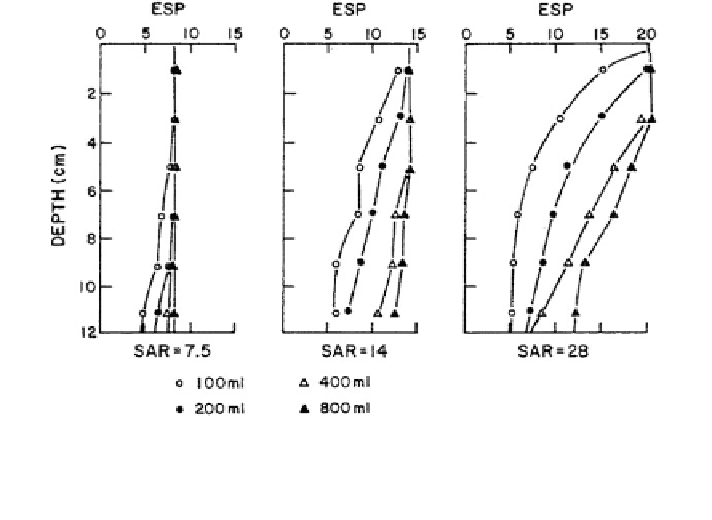
Fig. 8.26 Exchangeable sodium percentage (ESP) along a Burleson soil column, as a function of
the sodium adsorption ratio (SAR) of irrigation water. The values were obtained by percolating
the soil columns with sodic water (total electrolyte concentration of 11 mEq/L). Each curve
corresponds to a given applied volume of solution (Thomas and Yaron
1968
)
Heavy metal sorption on soil constituents may occur by one of the two
mechanisms: (1) in a two-step mechanism where a rapid chemical reaction takes
place on the surface of soil mineral followed by a long-time process recognized as
a film diffusion route; (2) a three-step mechanism including (a) rapid adsorption of
heavy metals on the external surface of the soil constituents, (b) delayed move of
the heavy metal from the external to internal sites by solid-state film diffusion, and
(c) binding and fixation of heavy metal at positions in the internal phase of the soil/
mineral particle.
Sparks (
2005
) illustrated these adsorption mechanisms by comparing kinetics of
some heavy metal sorption on a soil and various soil minerals. In this study, Ni, Pb,
and As sorption on kaolinite, pyrophylite, gibbsite, montmorillonite, and Mata-
peake soil was compared as shown in Fig.
8.27
. The sorption process was con-
trolled by both sorbent and sorbate properties. An example of the three-step
mechanism was demonstrated for As(V) adsorption on ferrihydrite where a great
part of applied contaminant was sorbed within the first 5 min followed by slow
sorption during *200 h (Fig.
8.27
c).
The desorption of heavy metals may exhibit an hysteretic pathway, the hys-
teresis being controlled by residence (contact) time with the sorbent. An example
of the residence time effect on desorption of Pb sorbed on Matapeake soil is given
in Table
8.7
. By extrapolating the data of Strawn and Sparks (
2000
), we can
assume that Pb adsorption under specific environmental conditions may become
irreversible on a lifetime scale.