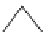Geoscience Reference
In-Depth Information
Fig. 1.3 Typical three-
layered phyllosilicate
structures (Sposito
1984
)
TETRAHEDRAL SHEET
O
2
−
Si
4
−
DIOCTAHEDRAL SHEET
X
b
−
M
m
+
their free energy. The most common inorganic structural units in clay minerals
present in the subsurface are the silica tetrahedron SiO
4
and octahedral complex
MX
6m-6b
composed of a metal unit (M
m+
) and six anions (X
b-
). Figure
1.3
shows
sheet structures formed by polymerization of these two structural units.
The architecture of a silicate layer results from SiO
2
coordination in which each
SiO
2
unit shares oxygen atoms with three neighboring SiO
4
groups, thus forming
rings containing six Si and six O atoms. Each ring joins the neighboring ring
through shared oxygen atoms. An additional structural element in layered silicate
is an octahedral sheet that contains cations in MO
6
coordination between the two
planes of oxygen atoms.
As a function of their structural properties, clays interact differently with
organic and inorganic contaminants. Two major groups of clay minerals are
selected for discussion here: (a) kaolinite, with a 1:1 layered structured alumi-
nosilicate and a surface area ranging from 6 to 39 m
2
/g (Schofield and Samson
1954
); and (b) smectites with a 2:1 silicate layer and a total surface area of about
800 m
2
/g (Borchardt
1989
).
1.1.2.1 Kaolinite
Kaolinite crystals in the subsurface are submicron sized and exhibit a platelike
morphology. They usually are found mixed with other layered structured minerals.
In a comprehensive review, Dixon (
1989
) summarizes the structural properties of
kaolinite.
This
mineral
is
composed
of
tetrahedral
and
octahedral
sheets