Geoscience Reference
In-Depth Information
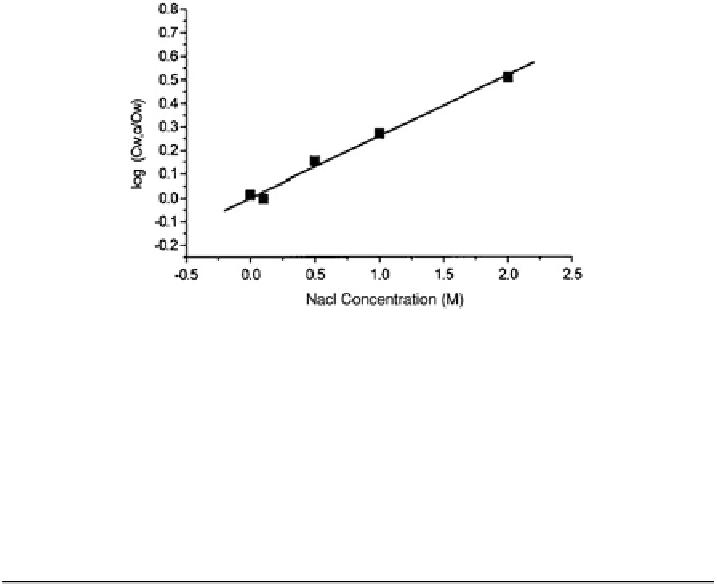
Fig. 8.24 Decrease in kerosene solubility in a NaCl aqueous solutions of various concentration
as result of the salting-out effect (Dror et al.
2000
). C
w,0
and C
w
denote concentrations at initial
maximum solubility and at various salinities
Table 8.4
K
salt
values for three tested drugs
Calculated
a
K
salt
Solute
Experimental K
salt
logK
ow
Phenytoin
0.191
2.08
0.198
Theophylline
0.100
-0.06
0.115
Cytosine
-0.005
-1.65
0.053
a
Based on K
salt
= 0.039 logK
ow
+ 0.117 (Ni et al.
2000
)
Pharmaceuticals and personal care products represent potential contaminants; we
consider it appropriate to include an example of the salting-out effect on drug
solubility in aqueous solutions. Ni et al. (
2000
) examined the relation between the
Setschenow constant (K
salt
) of a nonelectrolyte in a NaCl solution and the logarithm
of its octanol-water partition coefficient, log K
ow
. The relation K
salt
= A logK
ow
+
B, where A and B are constants, was tested for 15 compounds including a number of
drugs. The values of A (=0.039) and B (=0.117) were determined empirically from
the literature data for 62 organic compounds. This linear relationship provides a
simple and accurate method to predict the salting-out effect on organic compound
solubility and was used to determine the K
salt
for the drugs phenytoin, theophylline,
and cytosine; the results are presented in Table
8.4
.
When a NAPL is ''mixed'' with saline water, the coupled effects of salting out and
droplet formation can occur. Despite an expected decrease in concentration due to
the salting-out effect, a higher total organic concentration may be found. This
phenomenon is explained by formation of a pseudo ''oil-in-water'' emulsion, with
droplets of various sizes leading to increased apparent solubilities that may be up to
several times greater than those known theoretically. This phenomenon also con-
tradicts the salting-out effect. These droplets, which are not visible to the naked eye,
are observed by optical microscope and detected by gas chromatography analysis.