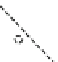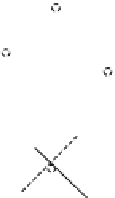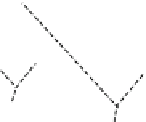Geoscience Reference
In-Depth Information
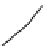
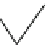
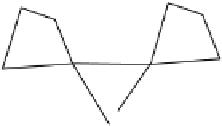
Fig. 1.1 The structure of
quartz (Dress et al.
1989
)
1
3
2
1
3
2
1
Si
quartz can be visualized as a spiral network of silica tetrahedra around the z-axis.
From Fig.
1.1
, it is seen that each tetrahedron is repeated in the network by a
rotation of 120 and a translation of c/3.
Quartz yields a characteristic X-ray pattern with well-defined peaks exhibiting a
d-spacing ranging between 0.426 and 1.182 nm (Dress et al.
1989
). Quartz is
distinguished from other silica polymorphs by a distinctive infrared (IR) absorp-
tion band at 692 cm
-1
, with two strong doublets at 798 and 780 cm
-1
and at 395
and 370 cm
-1
(Chester and Green
1968
).
Quartz in the subsurface usually is altered by in situ chemical and physical
weathering. Quartz appears as an anhydrous grain losing its prismatic form and
containing trace elements other than silicon and oxygen. Aluminum is the major
potential contaminant of the quartz mineral, but other trace elements such as Ti,
Fe, Na, Li, K, Mg, Ca, and H (OH) also are present (Dennen
1966
). Quartz grains
are in general rounded or have an angular morphology due to physical attrition. A
cleavage mechanism leads to the formation of flat grains when the quartz particles
are \100 lm (Krinsley and Smalley
1973
). Scanning electron micrographs of
quartz grains are presented in Fig.
1.2
.
The dense packing of the crystal structure and the high activation energy
required to alter the Si-O-Si bond contribute to the high stability of quartz (Stober
1967
). Quartz in the subsurface includes chemically precipitated forms commonly
associated with carbonates or carbonate-cemented sandstones (Dapples
1979
). In