Geoscience Reference
In-Depth Information
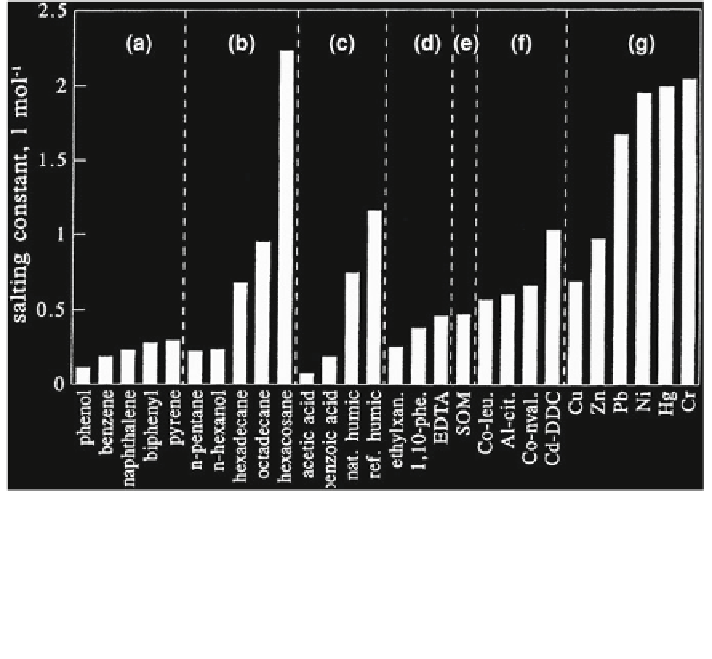
Fig. 6.4 Calculated, estimated, or apparent salting-out constants for various chemicals:
a selected aromatic compounds, b selected aliphatic compounds, c natural or surrogate ligands,
d anthropogenic ligands, e sediment organic matter (SOM), f transition metal complexes, g trace
metal complexes in the Mersey Estuary. Reprinted with permission from Turner A, Martino M,
Le Roux SM (
2002
) Trace metal distribution coefficients in the Mersey Estuary UK: Evidence for
salting out of metal complexes. Environ Sci Technol 36:4578-4584. Copyright 2002 American
Chemical Society
subsequently neutralize organic ligands, with the resulting neutral assemblage
possibly being salted out via electrostriction. This behavior has significant
implications for reactivity, availability, and transport of organic contaminants in
saline water bodies rich in potential organic ligands. Calculated, estimated, or
apparent salting constants for various chemicals are presented in Fig.
6.4
.
6.6 Apparent Solubility
Changes in the pH of subsurface aqueous solutions may lead to an apparent increase
or decrease in the solubility of organic contaminants. The pH effect depends on the
structure of the contaminant. If the contaminant is sensitive to acid-base reactions,
then pH is the governing factor in defining the aqueous solubility. The ionized form
of a contaminant has a much higher solubility than the neutral form. However, the
apparent solubility comprises both the ionized and the neutral forms, even though
the intrinsic solubility of the neutral form is not affected.