Geoscience Reference
In-Depth Information
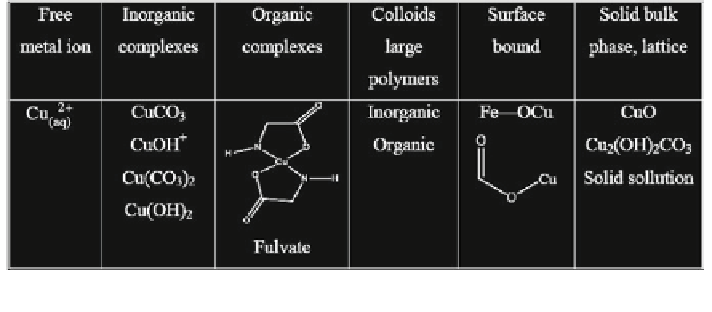
Fig. 6.3
Forms of occurrence of metal species in natural waters (Stumm and Morgan
1996
)
Humic and fulvic acids are the main natural ligands acting in the subsurface
aqueous solution. An example of a metal species that may occur in natural waters
as a result of potential inorganic and organic ligands is presented in Fig.
6.3
.Itis
difficult, however, to differentiate experimentally between dissolved and colloid
substances in subsurface aqueous solutions, and this should be considered when
considering the solubility of toxic trace elements in the subsurface system.
6.4 Cosolvents and Surfactant Effects
Many contaminants, such as pesticides and pharmaceuticals, reach the subsurface
formulated as mixtures with dispersing agents (surfactants). Such formulations
increase the aqueous solubility of the active compounds, and these surfactants
form nearly ideal solutions with the aqueous phase.
Addition of a cosolvent is an alternative mechanism to increase contaminant
solubility in an aqueous solution. When a contaminant with low solubility enters
an aqueous solution containing a cosolvent (e.g., acetone), the logarithm of its
solubility is nearly a linear function of the mole fraction composition of the
cosolvent (Hartley and Graham-Bryce
1980
). The amount of contaminant that can
dissolve in a mixture of two equal amounts of different solvents, within an aqueous
phase, is much smaller than the amount that can dissolve solely by the more
powerful solvent. In the case of a powerful organic solvent miscible with water, a
more nearly linear slope for the log solubility versus solvent composition rela-
tionship is obtained if the composition is plotted as volume fraction rather than
mole fraction.
Yalkowsky and Roseman (
1981
) and Rubino and Yalkowsky (
1987
) suggest the
following equations for relating solubility of a nonpolar solute (S
m
) in a binary
mixture of an organic solvent and water to that in pure water (S
w
):