Geoscience Reference
In-Depth Information
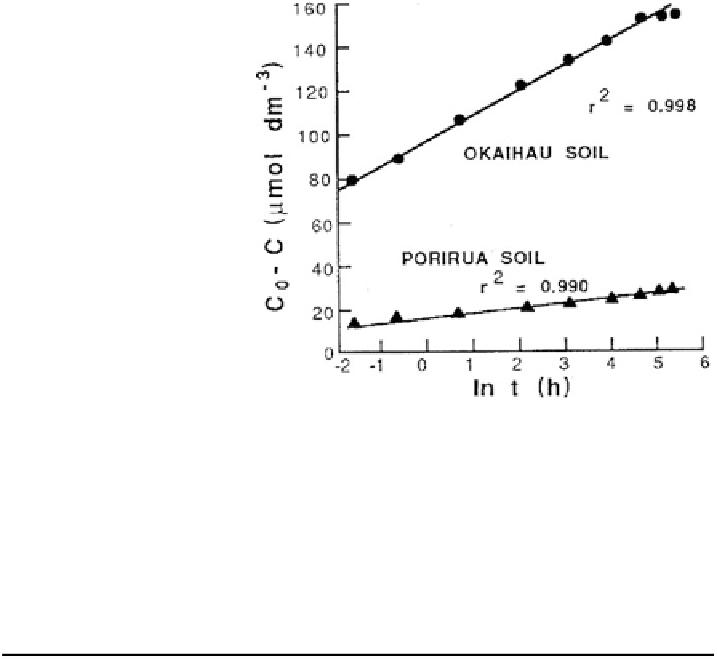
Fig. 5.4 Plot of Elovich
equation for phosphate (PO
4
)
sorption on two soils, where
C
0
is the initial concentration
added at time zero, and C is
the concentration in the soil
solution at time t (Chien and
Clayton
1980
)
Table 5.3 Apparent rate constants for the release of potassium from potassium minerals, as a
function of temperature (Huang et al.
1968
)
Mineral
Temperature
301 K
311 K
1.46 9 10
-2
3.09 9 10
-4
Biotite
9.01 9 10
-4
2.44 9 10
-4
Phlogopite
1.39 9 10
-4
4.15 9 10
-4
Muscovite
7.67 9 10
-5
2.63 9 10
-4
Microcline
5.4 Adsorption of Ionic Contaminants
Chapter 2
mentioned that the adsorption of charged ionic compounds on the solid
phase is a result of a combination of chemical binding forces and electric fields at
the interface. Here, we extend the discussion on this topic, focusing mainly on
aspects relevant to behavior of ionic contaminants in the subsurface environment.
Electrical neutrality on the solid surface requires that an equal amount of
positive and negative charge accumulates in the liquid phase near the surface. If
the surface is negatively charged, positively charged cations are electrostatically
attracted to the surface. Simultaneously the cations are drawn back toward the
equilibrating solution; as a result, a diffuse layer is formed and the concentration of
cations increases toward the surface. On the other hand, ions of the same sign
(anions) are repelled by the surface with diffusion forces acting in an opposite
direction. The overall pattern is known as a diffuse double layer (DDL). The
existence of a DDL was developed theoretically by Gouy and Chapman about
100 years ago and is an integral part of electric double layer theory.